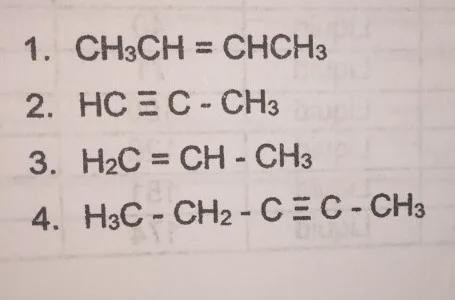
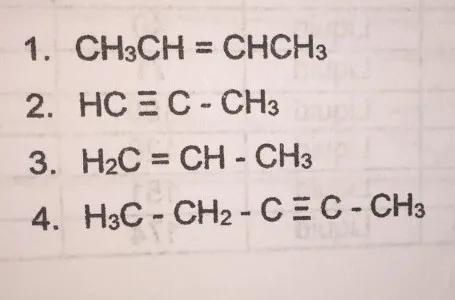
Answers
We have the next compounds
And we must classify them as alkane, alkene, alkyne.
We need to know that
- Alkane: They are the simplest hydrocarbons, containing only carbon and hydrogen held together by single bonds.
- Alkene: Alkenes are hydrocarbons that contain a double bond.
- Alkyne: Alkynes are hydrocarbons that contain a triple bond.
1. We can see that the structure has a double bond. So, it is an Alkene.
2. We can see that the structure has a triple bond. So, it is an Alkyne.
3. We can see that the structure has a double bond. So, it is an Alkene.
4. We can see that the structure has a triple bond. So, it is an Alkyne.
ANSWER:
1. Alkene
2. Alkyne
3. Alkene
4. Alkyne
Related Questions
Calculate the number of moles of magnesium, chlorine, and oxygen atoms in 8.30 moles of magnesium perchlorate,Mg(Cl04)2Express the number of moles of Mg, Cl, and O atoms numerically, separated by commas.moles of Mg, CI, O =
Answers
The question requires us to calculate the number of moles of each element (magnesium, chlorine and oxygen) in 8.30 moles of the compound magnesium perchlorate (Mg(ClO4)2).
From the molecular formula of magnesium perchlorate we can take the following information:
- in 1 mol of Mg(ClO4)2, there is only 1 mol of Mg;
- in 1 mol of Mg(ClO4)2, there are 2 moles of Cl;
- in 1 mol of Mg(ClO4)2, there are 8 moles of O.
With the information above, and knowing that the amount of Mg(ClO4)2 given by the question was 8.30 moles, we can calculate the amount, in moles, of each element:
1 mol Mg(ClO4)2 --------------- 1 mol Mg
8.30 mol Mg(ClO4)2 ---------- x = 8.30 mol Mg
1 mol Mg(ClO4)2 --------------- 2 mol Cl
8.30 mol Mg(ClO4)2 ---------- y = 2 x 8.30 mol Cl = 16.6 mol Cl
1 mol Mg(ClO4)2 --------------- 8 mol O
8.30 mol Mg(ClO4)2 ---------- z = 8 x 8.30 mol O = 66.4 mol O
Therefore, there are 8.30, 16.6 and 66.4 moles of Mg, Cl and O in 8.30 moles of magnesium perssufate.
True or false; The amount of energy required to heat 10 grams of water by 15 degreesCelsius is greater than that required to heat 10 grams of aluminum from15 degrees Celsius.
Answers
To answer this question we have to compare the specific heats of each substance.
The specific heat of a substance is the amount of energy required to make 1 gram of a substance increase its temperature by 1 °C. The greater the specific heat of a substance the more energy it will be required to heat it up.
The specific heat of water is 4.18J/g°C and the specific heat of aluminum is 0.897J/g°C.
It means that the amount of energy required to heat water is greater than the one to heat aluminum.
It means that the answer is TRUE.
12: from the following list, decide whether the following are Arrhenius bases or acids.•HCl•NaOh•HBr•H2SO4•NH4OH•H3PO4•HNO3•LiOH•Ba(OH)2•CsOH•HF•KOH
Answers
Answer:
The Arrhenius acids in the list are:
HCl, HBr , H2SO4 , H3PO4 , HNO3 , HF
The Arrhenius bases are:
NaOH , NH4OH , LiOH , Ba(OH)2 , CsOH and KOH
Explanation:
An Arrhenius acid increases the concentration of H+ ions while an Arrhenius acid increases the concentration of OH- ions
Thus, an Arrhenius acid would yield H+ ions on ionization while an Arrhenius base will yield OH- ions on ionization
The Arrhenius acids in the list are:
HCl, HBr , H2SO4 , H3PO4 , HNO3 , HF
The Arrhenius bases are:
NaOH , NH4OH , LiOH , Ba(OH)2 , CsOH and KOH
I need help with something please
Answers
Since both metals are in the same group in the periodic table and both non-metals are also in the same group in the periodic table, they have the same charge (metals = 1+; non-metals = 1-). So this makes the reaction easier for us:
NaCl + LiBr = LiCl + NaBr
Define hydrogen bonding and explain how hydrogen are bonding involved in the
transfer of genetic material.
Answers
Answer:
Hydrogen bonding is a special type of dipole-dipole attraction between molecules, not a covalent bond to a hydrogen atom. It results from the attractive force between a hydrogen atom covalently bonded to a very electronegative atom such as a N, O, or F atom and another very electronegative atom.
Explanation:
3. A 5.00 mole sample of oxygen gas has a pressure of 1.25 atm at 22℃. What is the volume of the gas?
Answers
The volume of a gas that has a pressure of 1.25 atm at 22℃ is 96.88L.
How to calculate volume?The volume of a gas can be calculated by using the following formula:
PV = nRT
Where;
P = pressure of the gas V = volume of the gasn = number of moles of the gasT = temperatureR = gas law constantAccording to this question, 5.00 mole sample of oxygen gas has a pressure of 1.25 atm at 22℃. The volume can be calculated as follows:
1.25 × V = 5 × 0.0821 × 295
1.25V = 121.0975
V = 96.88L
Therefore, 96.88L is the volume of the gas
Learn more about volume at: https://brainly.com/question/24189159
#SPJ1
How many milliliters of 0.165 M HCL are needed to neutralize completely 25.0 mL of 0.101 M Ba(OH)2
Answers
We have a neutralization between the HCl (acid) and Ba(OH)2 (base):
To calculate the mL asked, we need to use the next formula:
M1 x V1 = M2 x V2 (1)
We assign number 1 to HCl and number 2 to Ba(OH)2
Remember: M molarity and V volume
-----------------
Data:
We know M1 = 0.165 M (HCl) and M2 = 0.101 M, V2 = 25.0 mL.
-----------------
Procedure:
We clear V1 from (1):
V1 = M2 x V2/M1 = 0.101 M x 25.0 mL/0.165 M = 15.3 mL
Answer: V1 = 15.3 mL
Abed found fingerprints at a crime scene that match fingerprints that were taken from the suspect. What does this MOST likely mean?
A.
The suspect’s fingerprints match someone else’s.
B.
All 150 ridge characteristics are identical.
C.
The suspect touched something at the crime scene.
D.
At least three different points were a perfect match.
Answers
Answer:
C
Explanation:
Because the suspect touched something at the crime scene and thus he's fingerprint are matching
What is the molarity of ions in a 0.561 M solution of Ca(OH)₂
assuming the compound dissociates completely?
Answers
For each particle (molecule) of Ca(OH)2 that was present, assuming full dissociation of the complex, you would get two particles (ions). CaO + H2O equals Ca(OH)2 (2 particles)
A 0.561 M solution contains 0.561 moles of Ca(OH)2 per kilogram of solvent. Ions' molality would be 2 x 0.561m = 1.122m.
How is an ion's molarity determined?The most used unit for expressing solution concentration is molarity (M), which is calculated by dividing the solute concentration in moles by the volume of the solution in liters: M stands for moles of solute per liter of solution.
What does ion molality mean?The kilograms of solvent divided by the moles of ions in the solution is the molality. As an illustration, if You will have 1.0 molal concentration of sodium chloride, for instance, if 1.0 moles of sodium chloride are dissolved in 1.0 kilogram of solution.
To know more about molarity of ions visit:-
https://brainly.com/question/26263047
#SPJ13
What is the new volume in liters if 3500 mL of gas is cooled from 80 °C to 25 °C and pressure remains constant? I know the answer is 2.95 L. Please show your work.
Answers
Answer:
2.95 L
Explanation:
To find the new volume, you need to use the Charles' Law equation:
V₁ / T₁ = V₂ / T₂
In this equation, "V₁" and "T₁" represent the initial volume and temperature. "V₂" and "T₂" represent the final volume and temperature. Since you want your final volume in liters, you first need to convert 3,500 mL to L. Then, you need to convert the temperatures from Celsius to Kelvin. Finally, you can plug the given values into the equation and simplify to find "V₂".
V₁ = 3,500 mL / 1,000 = 3.5 L V₂ = ? L
T₁ = 80°C + 273 = 353 K T₂ = 25°C + 273 = 298 K
V₁ / T₁ = V₂ / T₂ <----- Charles' Law equation
(3.5 L) / (353 K) = V₂ / (25°C) <----- Insert values
0.00992 = V₂ / (298 K) <----- Divide left side
2.95 = V₂ <----- Multiply both sides by 298 K
What makes water a polar molecule? options:A) The equal forces between oxygen and hydrogenB) The liquid nature of waterC) The uneven bend in the bond between oxygen and hydrogenD) The ionic bond between oxygen and hydrogen
Answers
The polarity of a molecule is given by the difference in charges, hydrogen has a slightly positive charge and oxygen has a negative charge. Its electronegativity difference between hydrogen and oxygen is less than 1.7 making the bond a covalent bond.
That said, we can rule out options A and D. Now, the state of matter does not affect the polarity of the molecule, so option B will also be incorrect.
The correct option will be C, since there is a difference in charges that makes water polar.
Answer: C) The uneven bend in the bond between oxygen and hydrogen
Question 7 of 10What makes up an ionic compound?O A. A positive cation and a negative anionB. A negative anion and a negative anionOC. A positive anion and a negative cationOD. A positive cation and a positive anionаSUBMIT
Answers
A ionic compound is made of two ions, one with positive charge and the other one with a negative charge.
The ion with positive charge is known as cation and the one with negative charge is known a anion.
It means that the correct answer is A. A positive cation and a negative anion.
Consider the balanced thermochemical equation given below: W(s) + 3 H2O(g) → WO3(s) + 3 H2(g) ∆Η° = –125.9 kJ How many grams of H2 were produced when 377 kJ of heat was produced?
Answers
Answer:
Explanation:Consider the balanced thermochemical equation given below: AH = -125.9 kJ W(s) + 3 H2O(g) WO3(s) + 3 H2(g) If a mass of 94.6 g H2 is produced, what is the enthalpy change? Express your answer in kJ. Express your answer to the correct number of significant figure, in scientific notation and include the unit with
Potential Molecular TheoryThere are 500 grams of Kl in a volumetric flask. It is dissolved in 1 liter of water. What is the molarity?3.141 M3.012 M0.500 M500.000 M20A precipitate is formed when two solutions are mixed. This would only occur if
Answers
Molarity or molar concentration is a measure of the concentration of a chemical species, of a solute in a solution, using as units, number of moles, and volume in liters. The formula for Molarity is:
M = n/V
Where:
n = number of moles
V = volume in Liters, 1 Liter
But we need to find the number of moles, we will do so by using its molar mass, KI = 166g/mol and the given mass in the question, 500g grams
166g = 1 mol
500g = x moles
x = 3.012 moles
Now we can use the Molarity formula
M = 3.012/1
M = 3.012 M, this is the molar concentration of the solution, 2
What is the percent yield if 15.5 g SO2 is obtained from the reaction of 42.5 g of O2 with excess ZNS according to the following equation 2ZnS (s) + 3O2 -> 2ZnO (s) + 2 SO2(g)
Answers
Answer:
Percent yield = 27.3%.
Explanation:
First, let's write the chemical equation:
[tex]2ZnS+3O_2\rightarrow2ZnO+2SO_2.[/tex]The limiting reactant, in this case, would be O2 because we have an excess of ZnS. So, we have to convert 42.5 g of O2 to moles. Remember that the molar mass of O2 is 32 g/mol (you can calculate the molar mass of a compound using the periodic table). The conversion will be:
[tex]42.5\text{ g O}_2\cdot\frac{1\text{ mol O}_2}{32\text{ g O}_2}=1.33\text{ moles O}_2.[/tex]With this value, we're going to find the number of moles of SO2 produced by 1.33 moles of O2. You can see in the chemical equation that 3 moles of O2 reacted produces 2 moles of SO2, so the calculation would look like this:
[tex]1.33\text{ moles O}_2\cdot\frac{2\text{ moles SO}_2}{3\text{ moles O}_2}=0.887\text{ moles SO}_2.[/tex]The next step is to find the mass of SO2 based on its number of moles and the molar mass of SO2 which is 64 g/mol, like this:
[tex]0.887\text{ moles SO}_2\cdot\frac{64\text{ g SO}_2}{1\text{ mol SO}_2}=56.8\text{ g SO}_2.[/tex]And finally, we replace the values that we have in the formula of percent yield:
[tex]\%\text{ yield }=\frac{experimental\text{ yield}}{theoretic\text{al yield}}\cdot100\%.[/tex]Our experimental yield is the mass that we obtained of SO2 which is 15.5 g and the theoretical yield is the mass that we found through stoichiometry which is 56.8g:
[tex]\%\text{ yield}=\frac{15.5\text{ g}}{56.8\text{ g}}\cdot100\%\approx27.3\%.[/tex]The percent yield of this reaction would be 27.3%
Define electronegativity.
A neutral atom has high electronegativity. Describe what happens to this atom during ionic bond formation.
No trolls, fake answers, copied answers
Answers
Electronegativity is defined as the ability of an element to accept electrons to its shell during a chemical reaction for the formation of a stable bond structure.
What is a neutral atom?A neutral atom is the atom that contains an equal amount of electrons and protons which means that is has a zero net charge. Example is sodium.
Electronegativity is defined as the ability of an element to accept electrons to its shell during a chemical reaction for the formation of a stable bond structure.
During ionic bond formation, the elements involved tries to obtain an octet structure by either donating or accepting electrons.
Alkali metals( such as the neutral element of sodium? have the lowest electronegativities, while halogens have the highest.
Learn more about electronegativity here:
https://brainly.com/question/24977425
#SPJ1
Draw electron dot structures for the following substances: a. C12, b. CO, c. CO2 d. NH3, e. CC14, f. H2O
Answers
When drawing an electron dot diagram, you need to check the exact number of electrons that an atom has in its shell, only the valence electrons must be drawn
What element has the most similar property as fluorine
Answers
Answer:
Chlorine and Bromine are also halogens so they are similar to fluorine
How much water needs to be added to 65 mL of 3.0 M stock solution to produce a 1.0 M diluted solution?
Answers
To answer this question, we have to use the dilution rule, that is represented by the following equation:
[tex]C1V1=C2V2[/tex]Where C1 and C2 are the initial and final concentrations and V1 and V2 are the initial and final volumes.
Solve the equation for V2 to find the final volume:
[tex]\begin{gathered} V2=\frac{C1V1}{C2} \\ V2=\frac{3.0M\cdot65mL}{1.0M} \\ V2=195mL \end{gathered}[/tex]The final volume of the solutions has to be 195mL. Since we already have 65mL, we need to add 130mL of water to have 195mL in total.
It means that the correct answer is 130mL.
The chemical reaction of hydrogen with oxygen produces water.
2H₂(g) + O₂(g) → 2H₂0 (g)
a. How many moles of O₂ are required to react with 2.6 moles of H₂?
b. How many moles of H₂ are needed to react with 5.0 moles of O₂?
Answers
Answer: a
explanation: Make a ratio of the number of moles and do the calculations. Do you get it?
How many Chiral centers are in coibacin B
Answers
It is to be noted that Coibacin B has three Chiral Centers. A chiral center is a molecule atom that is bound to four separate chemical species, permitting optical isomerism.
What is Coibacin B?Coibacin B is a natural substance with a substantial anti-inflammatory effect and possible utility in the treatment of leishmaniasis, a parasitic illness.
It should be noted that Leishmaniasis is a parasite infection that may be found in the tropics, subtropics, and southern Europe. It is considered a neglected tropical illness (NTD).
Infection with Leishmania parasites, which are disseminated by the bite of phlebotomine sand flies, causes leishmaniasis.
Learn more about Chiral Centers:
https://brainly.com/question/28095208
#SPJ1
How many moles of carbon dioxide are formed when reacting with 36 moles of oxygen?
Answers
1) Chemical equation
[tex]CH_4+2O_2\rightarrow CO_2+2H_2O[/tex]2) Moles of CO2 produced oxygen reacts
The molar ratio
2 mol O2: 1 mol CO2
[tex]molCO_2_{}=36molO_2\cdot\frac{1molCO_2}{2molO_2}=18molCO_2[/tex]If 36 mol O2 reacts, it will produce 18 mol CO2.
.
6.5 grams of zinc reacted with hydrochloric acid. Calculate how many grams of hydrogen were released. a) 0.1 g. b) 0.2 g. c) 1.0 g. d) 2.0 g.
Answers
0.2 grams
ExplanationsThe balanced reaction between zinc and hydrochloric acid is given as:
[tex]Zn+2HCl\rightarrow ZnCl_2+H_2[/tex]Given the following parameters
Mass of Zinc = 6.5grams
Determine the mole of Zinc
Mole of Zn = mass/molar mass
Mole of Zn = 6.5/65.38
Mole of Zn = 0.0994moles
According to stoichiometry, 1 mole of Zinc produced 1 mole of hydrogen gas, hence the mole of hydrogen gas required will be 0.0994moles
Determine the mass of hydrogen gas produced
[tex]\begin{gathered} Mass\text{ of H}_2=mole\times molar\text{ mass} \\ Mass\text{ of H}_2=0.0944moles\times2.016 \\ Mass\text{ of H}_2=0.19g\approx0.2grams \end{gathered}[/tex]Hence the mass of hydrogen that were released is 0.2 grams
I need help with filling out the cubes and emery’s, basically the Application: Assigning Oxidation numbers (shown in the photo)
Answers
According to the explanation about oxidizing and reducing agents from the previous session, we will find the answers to this question as well
Oxidation numbers are mostly found in previously made tables, there are plenty of tables where you can see the oxidation numbers for almost every element, a few rules to have in mind is H is usually +1, O is -2, Halogens are -1, group 1A metals are +1, transition metals are the ones that will shift a lot
1. Sn + 2 HCl -> SnCl2 + H2, this is the properly balanced equation
REACTANTS
Sn = 0, since it's a lone element in the reaction we assume its charge is 0 because is stable
H = +1
Cl = -1, it's a halogen
PRODUCTS
Sn = +2, we see now that Sn was oxidized and now has a positive charge
Cl = still -1
H2 = 0, was reduced, now with a 0 charge
2. 2 NaBr + Cl2 -> 2 NaCl + Br2
REACTANTS
Na = +1, metal from group 1A
Br = -1, halogen
Cl2 = 0 stable element
PRODUCTS
Na = +1
Cl = -1, it will be reduced
Br = 0, it will be oxidized
3. SiCl4 + 2 Mg -> 2 MgCl2 + Si
REACTANTS
Si = +4
Cl = -1
Mg = 0
PRODUCTS
Mg = +2, it will be oxidized
Cl = -1
Si = 0, it will be reduced
a sample of a gas is at STP and it’s temperature remains constant. if the pressure decreases what will happen to the volume
Answers
Explanation
There are said to have two different conditions of a gas.
First condition is STP which means: pressure = 1 atm and temperature (absolute) = 0 °C + 273 = 273 K
-------------
It is said that the temperature remains constant, therefore we can apply Boyle's law which states that the pressure of a gas tends to decrease as the volume of the container increases.
What consists of water molecules that have escaped or evaporated from the body of water
Answers
Water molecules that have escaped or evaporated from the body of water then water get excited and they begin to gain kinetic energy
A water molecule has a three atom two hydrogen and one oxygen atom and that's why water is sometime refred to as H₂O and a single drop of water contain billion drop of molecule and evaporation is the process in which given to the change that occurs to a substance that changes it from its liquid state to a gaseous state
When water get evaporates the molecule of water in a glass will get excited and they begin to gain kinetic energy and vigorously hit the wall of glass
Know more about evaporation
https://brainly.com/question/7481738
#SPJ1
Of the following EM waves, which has the highest frequency?A.X-raysB.Ultraviolet lightC.MicrowavesD.Infrared light
Answers
Answer
A. X-rays
Explanation
There are seven regions in the electromagnetic spectrum (EM) which in order of lowest to highest frequency are:
Radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays.
Therefore, of the following EM waves given in the options, the one that has the highest frequency is A. X-rays
Synthesize Information If you calculate the percent composition of elements in a compound, is there enough information to determine the empirical formula for the compound? If yes, how? If no, improve the model by identifying and explaining additional information needed to identify the compound.
Answers
If you only have the percent composition of each element in a give compound, you can't determine yet the empirical formular for the compound. The problem is, the percent composition only gives relative numbers for each element. What can be determined is the empirical stochiometry of one element relative to the others.
For example, supose you have a compound with element X and Y, with the percent composition you can determine how much X you have relative to how much Y you have. Say you determine that for each 2 X you have 3 Y, the formula can be X₂Y₃ but it can also be X₄Y₆ or even othre possibilities.
To determine the empirical formula you need to know how many of one of the elements you have in each compound. A common way of getting the empirical formula is to use the percent composition and, in addition, the molar mass of the compound, which will make it possible to get the empirical formula from the relative ratios of each element you have.
In an experiment 25 grams of chloroform and 25 grams of chlorine were mixed. Which is the limiting reagent? I picked C but I’m not sure if I’m correct
Answers
In this question, we have to find the limiting reactant based on the following reaction:
CHCl3 + Cl2 -> CCl4 + HCl
We have:
25 grams of CHCl3
25 grams of Cl2
The molar ratio between these two compounds is 1:1, 1 mol of CHCl3 for 1 mol of Cl2
Now we have to find the number of moles of each reactant, let's start with CHCl3, we will use its molar mass, 119.38g/mol:
119.38g = 1 mol
25g = x moles
119.38x = 25
x = 25/119.38
x = 0.209 moles of CHCl3
According to the molar ratio, if we have 0.209 moles of CHCl3, we will also have 0.209 moles of Cl2, which has a molar mass of 70.9g/mol
Let's find the mass of 0.209 moles of Cl2:
70.9g = 1 mol
x grams = 0.209 moles of Cl2
x = 14.81 grams
We only need 14.81 grams of Cl2 to react with 25 grams of CHCl3, since we have more Cl2 than we actually need, this makes Cl2 the excess reactant, and CHCl3 will be the limiting reactant. Letter C
If I have 10.0g of Mg, what is my theoretical yield of MgCl2
Answers
Explanation:
First, let's write the balanced equation of formation of MgCl2 from Mg and Cl2:
Mg + Cl2 → MgCl2
Now let's transform 10.0g of Mg into moles, using the following formula: moles = mass/molar mass
molar mass of Mg = 24.3 g/mol
moles = 10/24.3
moles = 0.412 moles
Now we use the equation ratio between Mg and MgCl2 to find the quantity of MgCl2 in moles.
1 mole of Mg produces 1 mole of MgCl2.
So 0.412 moles of Mg produces 0.412 moles of MgCl2.
Now we transform 0.412 moles of MgCl2 into mass using the following formula: mass = moles x molar mass
Molar mass of MgCl2: 24.3 + (2*35.45) = 95.21 g/mol
Mass = 0.412 * 95.21
Mass = 39.2 g
Answer: The theoretical yield of MgCl2 is 39.2 g.