Answers
Answer:
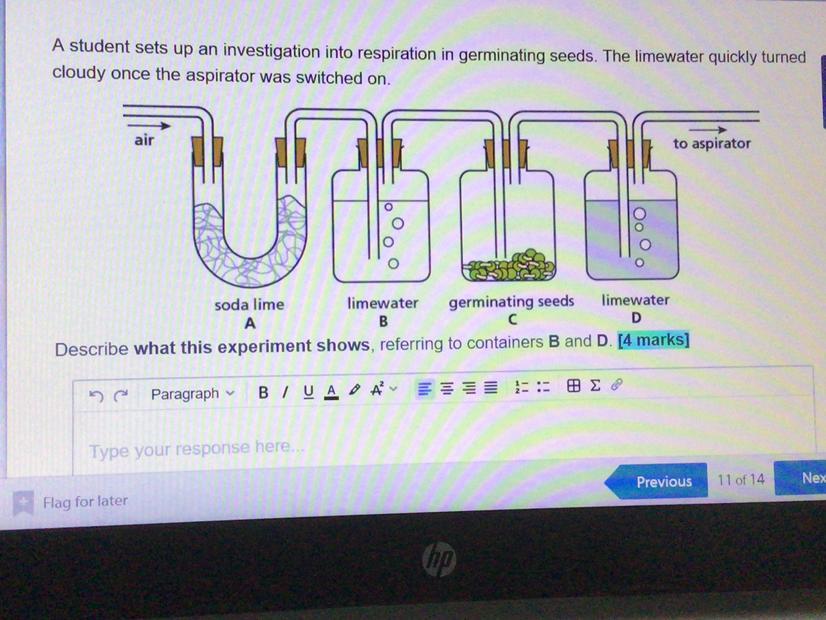
This shoes the soda lime defusing to limewater turning into germinating seeding which defuses back to limewater
Explanation:
Related Questions
Plowing is an example of what Energy?
a. Kinetic Energy
b. Potencial Energy
Answers
Answer:
mechanical or kinetic eg hammer and nails
HELP ASAPP JUST TELL ME WHAT TO PUT IN THE MISSING SPACES words(cell,microorganisms,micrometer,microscopic,molecule)
Answers
Answer:
microorganisms, molecule, microscopic, cell, micrometer
Explanation:
If you find my answer helpful
Pls consider marking it as Brainliest! It would mean a lot!
What is the effect of tap water, sea water, and rainwater on the rusting rate of iron?
(Please i need an answer π-π)
Answers
Answer:
The more acidic the solution the faster it rusts. More Na = more rust
Explanation:
The transition metals (with the exception of Zn, Cd and Hg) are very much hard and have low volatility. Why Zn ,Cd and Hg has low melting points. (a) These have all their electrons paired. (b) These have maximum number of unpaired electrons. (c) These have very strong metallic bonding. (d) These have more metal-metal bonding.
Answers
Answer:
Zn ,Cd and Hg has low melting points because - These have all their electrons paired.
Explanation:
These have all their electrons paired -: All the electrons in the d-subshell are combined with Zn , Cd and Hg. The metallic bonds present in them are, therefore, weak. This is why they have low points of melting and bolting. therefore , this statement is true. These have maximum number of unpaired electrons. Since , these transition metals have all electrons paired , so this statement is incorrect.These have very strong metallic bonding -: Zn, Cd and Hg d-orbital are completely filled. They have poor metallic bonding and less compact packing due to their fully filled d-orbitals, so all of them are volatile in nature. Therefore, this statement is also not valid .These have more metal-metal bonding. -: Since , their metallic bond is weak , so this statement is not true .Hence , the correct option is A (These have all their electrons paired).
Why MgO will form after Mg reacting with CuO? (mcq)
Mg forms positive ions and O forms negative ions
Mg is more reactive than Cu
CuO have loose bonding
Answers
Answer:
Mg is more reactive than Cu
Explanation:
In the reaction between Mg and CuO to form MgO, MgO will form because Mg is more reactive than Cu.
This kind of reaction is called a single replacement or single displacement reaction.
The replacement of a metallic ion in solution by a metal atom higher in the activity series than the metal in solution falls into this category of reactions. The element Mg is higher than Cu in the activity series. The higher an element is in the activity series, the more reactive they are. Since Mg is more reactive, it will displace the Cu from CuO.True or false
Each family represents the number of energy levels present in an atom of the element.
Answers
Answer:
true because of the elements
Write the word equations for the following balanced chemical equations.
a. Zn + 2HCl → ZnCl2 + H2
b. 2503 2502 + O2
Answers
Answer:
21
Explanation:9+10
What’s this called please help
Answers
Answer:
the answer is a linear
Explanation:
heres a graph different ones at this link
what is 1.23 x 10^-3 in standard notation
Answers
Answer:
=0.00123
Explanation:
Look at the attachments below
Hope this helps (:
Answer:
0.00123
Explanation:
Standard notation is the normal way of writing numbers. Examples include 1, 2, and 10. The number 1.23 x 10^-3 is written in scientific notation. The decimal goes after the first nonzero integer and it is multiplied by a power of 10. The power or exponent attached to the 10 tells you how many places over you need to move the decimal to get back into scientific notation. Examples include 1.00 x 10^2 (representing 100 in standard form because you would move the decimal two places to the right.), 2.0 x 10^1 (representing 20 in standard form because you would move the decimal one place to the right), and 3.0 x 10^-4 (representing 0.0003 in standard form because you would move the decimal four places to the left since it is a negative exponent).
The negative (-3) exponent in 1.23 x 10^-3 indicated to move the decimal three places to the left. If it was positive, you would move it three places to the right.
In 1.23 x 10^-3 move the decimal to the left 1 place to get:
0.123
two places to get:
0.0123
and a third place to get:
0.00123
The final answer is 0.00123
the ability for a substance to rust is
a. oxidation
b.rustability
c.magnetic attraction
d.reactivity
Answers
Answer:
i think it is letter b. rustability but not so sureeee
The density of air at STP is 1.285 g/L. Which of the following cannot be used to fill a balloon that will float in air at STP?
Answers
NO can't be used to fill a balloon
Further explanationConditions at T 0 ° C and P 1 atm are stated by STP (Standard Temperature and Pressure). At STP, Vm is 22.4 liters/mol.
Answer options that need to be added :
a. Ne
b. NO
c. NH₃
d. CH₄
e. HF
will float in air ⇒ element or compound to fill the balloon, its density must be less than < 1.285 g/L
We can use the ideal gas formula ta find density :
[tex]\tt \rho=\dfrac{P.MW}{RT}[/tex]
Because at STP, then the constant value is
[tex]\tt \rho=\dfrac{1~atm\times MW}{0.08205\times 273.15~K}\\\\\rho=0.0446\times MW[/tex]
So that the density is determined from the MW(molecular weight) of each element or compound
a. NeAr = 20.1797 g/mol
[tex]\tt \rho=0.0446\times 20.1797=0.9~g/L[/tex]
b. NOMW=30.006 g/mol
[tex]\tt \rho=0.0446\times 30.006=\boxed{\bold{1.338~g/L}}[/tex]
c. NH₃MW=17.0306 g/mol
[tex]\tt \rho=0.0446\times 17.0306=0.760~g/L[/tex]
d. CH₄MW=16.04 g/mol
[tex]\tt \rho=0.0446\times 16.04=0.715~g/L[/tex]
e. HFMW=20.01 g/mol
[tex]\tt \rho=0.0446\times 20.01=0.892~g/L[/tex]
25 POINTS
Boyd takes additional measurements of the mass of product formed in a reaction. He uses a balance that has smaller graduations than the first balance he used.
What is Boyd most likely trying to change?
Options:
He is trying to increase his accuracy but not his precision.
He is trying to increase his precision but not his accuracy.
He is trying to decrease his precision and increase his accuracy.
He is trying to increase his precision and decrease his accuracy.
Answers
Answer:
The correct option is A: "He is trying to increase his accuracy but not his precision"
Explanation:
Accuracy of measurement is when the value of the measurement is close to the actual value of that measurement while precision is when the experiment is repeated using the same instrument but obtaining different values.
When Boyd changed the weighing balance in his experiment to a smaller one, the smaller weighing balance has better accuracy (smaller graduations) than the initially used one because the smaller weighing balance will provide a value close to the actual value of the mass of the product. If he had, however, repeated the experiment without changing the weighing balance, he would have sought to increase his precision.
From the description above, it can be said that Boyd was trying to increase his accuracy but not his precision
Answer:
The correct option is A: "He is trying to increase his accuracy but not his precision"
Explanation:
right on EDGE 2021
Based on the article "Will the real atomic model please stand up?," describe what Dalton's theory states about a molecule of water. Dalton's theory about compounds tells us that all water molecules have different kinds of atoms, two hydrogen atoms for every one oxygen atom.
Answers
Answer:
Follows are the solution to this question:
Explanation:
The characterization with water molecules would be that light waves are made up of 2 different types of atoms (2 hydrogen and 1 oxygen atoms), as per the Dalton theory. There are many multiple times as many atoms of hydrogen as oxygen atoms in each water molecules. For every two hydrogen atoms, all water molecules have one oxygen atom.
Answer:
Sample Response: Dalton’s theory about compounds tells us that all water molecules have different kinds of atoms, two hydrogen atoms for every one oxygen atom.
Explanation:
What are the benefits and dangers of the Iron element in our daily life?
Answers
N2+H2》NH3
is it balanced or unbalanced
Answers
N2 + 3H2 -> 2NH3
Answer question number 3
Answers
Answer:
A
Explanation:
A mixture is formed when two or more substances are physically mixed together. A compound is formed when two or more substances are chemically combined through a chemical reaction.
What is the correct name for the compound P406?
A. Phosphoric acid
B. Tetraphosphorus hexoxide
C. Phosphorus (IV) oxide
D. Phosphorus oxide
Answers
Answer:
Phosphorus trioxide
Explanation:
Answer:
Tetraphosphorus hexoxide
Explanation:
Give brainliest please
what unit of measure would i use to measure the width of my fingernail A millimeters
B centimeters C meters D kilometers
E grams
Answers
Because the width of fingernails are very small and millimeters is the smallest unit of measurement listed.
Molecules in a liquid are:
closer together than in a gas
moving more quickly than in a solid
moving more slowly than in a gas
all of the above
Please help!!!
Answers
Answer:
all of the above
Explanation:
All of the above
Explanation:
compound name of SO2
Answers
Refer to your periodic table:
Which of the following has the smallest atomic radius?
Bromine
Astatine
Iodine
Fluorine
Answers
iondine
Explanation:
bromine has 185pm, astatine haa 200, iondine has 140 and lastly fluorine has 147. so iondine has the smallest atomic radius
8. Describe how crystals of copper sulphate are prepared.
Answers
Answer:
Take a cupful of water in a beaker and add a few drops of dilute sulphuric acid. Heat the water. When it starts boiling, add copper sulphate powder slowly while stirring continuously. ... Crystals of copper sulphate will be seen at the bottom of the beaker.
Hope it helps
A dull metal object has a density of 8.8 G/ML and a volume of 20 ML calculate the mass
Answers
Answer:
Mass = 0.000176 gram
Steps:
m = V × ρ
= 20 milliliter × 8.8 gram/cubic meter
= 2.0E-5 cubic meter × 8.8 gram/cubic meter
= 0.000176 gram
Explanation:
Which is the IUPAC name for NO?
nitric oxide
binitrogen oxide
dinitrogen dioxide
nitrogen monoxide
Answers
The IUPAC name for NO is nitric oxide. The correct option is A.
What is IUPAC naming?The International Union of Pure and Applied Chemistry (IUPAC) is a global organization that represents chemistry as well as related sciences and technologies.
The chemical compound's identity is represented by its common and IUPAC names, which differ from one another. Every chemical compound has multiple names.
Complete the following step-by-step response: The IUPAC nomenclature is a standardized name given to organic compounds in accordance with official naming rules.
Nitric oxide, also known as NO, is a colorless gas. It is one of the most important nitrogen oxides.
Nitric oxide is a free radical, which means it has an unpaired electron, which is sometimes represented in its chemical formula by a dot.
Thus, the correct option is A.
For more details regarding IUPAC, visit:
https://brainly.com/question/16631447
#SPJ6
Answer:
D: Nitrogen Monoxide
Explanation:
There's one Nitrogen atom and one Oxygen atom
A car travels 45 miles per hour. What is this speed in feet per hour?
Answers
Answer:
64
hopeeeeeeeeeeeeeeeeeeeeeeeeeeeeeeeeeeeeee
why is lithium used in the body?
Answers
Answer:
11
Explanation:
What is the relationship between the latitude and hours of daylight
Answers
Answer:
The tilt of the Earth's axis also defines the length of daylight.Daylight hours are shortest in each hemisphere winter. Between summer and winter
solistice the number of daylight hours decreases and the rate of decrease is larger the higher the latitude.The fewer sunlight hours the colder nights.
3. In an exercise to teach students how to use and analytical balance, the instructor
gives a student a quarter which has been pre-weighed as 5.6026 g. The weight that the
student obtains for the same quarter is 5.6013 g. What is the percent error in the
students reading?
Answers
Answer:
The percent error is 0.023%.
Explanation:
Pre-weighed of a quarter is 5.6026 g.
The weight that the student obtains for the same quarter is 5.6013 g.
We need to find the percent error in the students reading. It is given by the formula as follows :
[tex]\%=\dfrac{|\text{original value-calculated value}|}{\text{original value}}\times 100[/tex]
Putting all the values, we get :
[tex]\%=\dfrac{5.6026 -5.6013 }{5.6026 }\times 100\\\\\%=0.023\%[/tex]
So, the percent error is 0.023%.
Why is the nitrogen cycle considered a closed system?
Answers
Answer:
because nitrogen is fixed by bacteria and assimilated by plants
Explanation:
Answer:
It is a considered a closed system because nitrogen is fixed by bacteria and is taken in by plants.
Explanation:
Which of the following is NOT an example of a chemical change? *
O Corrosion
O Grinding
O Combustion
O Rust