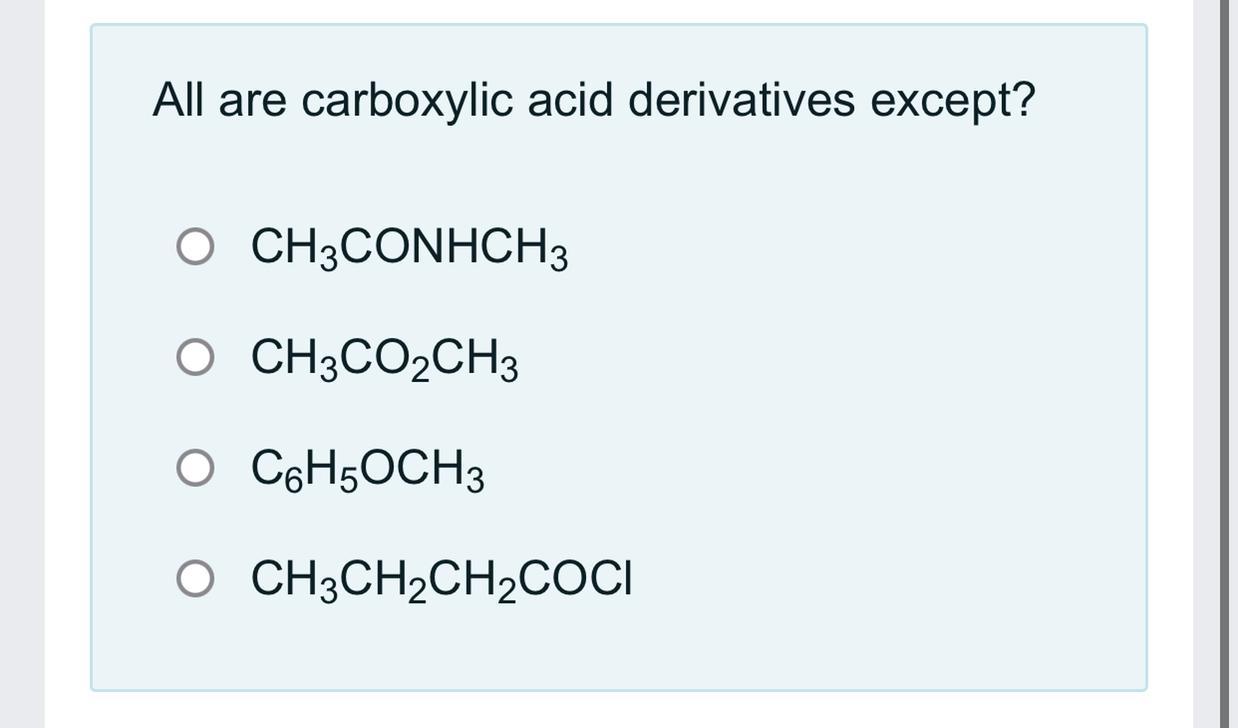
Answers
Answer: CHOCH
The third one
Explanation:
Related Questions
PART 1Select Introduction, and for the following unbalanced reactions found in the sim provide the missing coefficients.CoefficientReactant1CoefficientReactant2CoefficientProduct1CoefficientProduct2N2+H2HONH3H2O2COz
Answers
They give us the reactions with their respective products and reactants. To balance a reaction we must bear in mind that matter is neither created nor destroyed, it only transforms. So the mass that we have in the reactants must be the same in the products. We verify this by counting the atoms of each element on each side of the reaction.
In the first reaction we have nitrogen and hydrogen. We have 2 nitrogens and 2 hydrogens in the reactants. We start off-balanced in nitrogen, so we place coefficient 2 in the NH3 molecule to get 2NH3. Now that we have 2 nitrogens and 6 hydrogens in the products, we must balance the hydrogen in the reactants. For that, we place the coefficient 3 in the H2 in such a way that there will be 6 hydrogen atoms in the reactants. The equation is balanced and will be:
[tex]N_2+3H_2\rightarrow2NH_3[/tex]We do this same procedure for the other two equations. We count the atoms, we put the coefficients so that the number of atoms is conserved and we count again until the number of atoms of each element is the same on each side of the reaction. For the other two reactions we have:
[tex]2H_2O\rightarrow2H_2+O_2[/tex][tex]CH_4+2O_2\rightarrow CO_2+2H_2O[/tex]Andrew wants to become a certified forensic pathologist. What is MOST likely to be true about his path to achieve this goal?
A.
It is fairly easy and doesn’t take a long time.
B.
There is no required residency or clinical fellowship.
C.
It requires a medical degree like a regular doctor.
D.
There are no schools that are currently offering this training.
Answers
Answer:
A
Explanation:
it is easy and doesn't take a long time
HELP ASAP PLEASE. A legend is a traditional story that explains something in real life. Sometimes they are accepted as true, but sometimes they are fantastical and just plain fun. There are many legends that surround the North Star explaining its creation as well as why it is stationary.
Take some time to research some of the legends of the North Star. After you have had a chance to read several of them, create your own fantastical story of the North Star. It can be how it came to be or explain why it is stationary.
Answers
A legend of the North star goes as follows:
Once upon a time, when God was creating the universe, the curious star Tam once he learned that God was humans on earth, he began visiting humans. He became fond of one human. But each time he had to return to the sky, the human could not see him again. Therefore, he decided to remain stationary so that the human can always locate him.What is the North Star?The North star is a star that sits directly above the North pole of the earth. It is also known as Polaris.
The North star is especially significant in that the North star is an ever-present star in the skies. It neither rises nor sets unlike other stars in the skies.
Because of this special feature of the North star, it serves as a guiding beacon in the skies for travelers. Also, several legends have been told about the North star.
Learn more about the North star at: https://brainly.com/question/1130377
#SPJ1
9 mol of A how many moles of C are formed upon complete reaction of
Answers
Answer:
C = 9 mol
Explanation:
Reaction: A + 2B ---> C
We want to find the moles of C.
Given: moles of A = 9 mol.
To solve this problem we will need to use stoichiometry.
A is the limiting reagent because it has a coefficient of 1 and B has a coefficient of 2.
The molar ratio between A and C is 1:1 therefore for every 9 moles of A that react, 9 moles of C will be produced.
Write the two half-reactions that occur in this electroplating process. Be sure to include the states of the elements or ions.
Answers
Answer:
Explanation:
As the cell has a copper strip like the anode and a coin or nail (we can consider this like it is nickel) like the cathode, we can write both half-reactions:
• Copper half-reaction,:
[tex]Cu_{(s)}\leftrightarrows Cu_{(aq)}^{2+}+2e^-[/tex]In the copper anode, the oxidation reaction takes place. This is the positive terminal.
• Nickel half-reaction,:
[tex]Ni_{(aq)}^{2+}+2e^-\leftrightarrows Ni_{(s)}[/tex]In the nickel cathode, the reduction reaction takes place. This is the negative terminal.
The direction of the electron flow always go from the anode to the cathode.
Isopropyl alcohol is mixed with water to produce 32.0% (v/v) alcohol solution.How many milliliters of each component are present in 655 mL of this solution? Assume the volumes are additive.Alcohol: _____________ mL Water: __________ mL
Answers
Isopropyl alcohol + water = Solution
Solute = Isopropyl alcohol
Solvent = water
% V/V = mL of solute / 100 ml of solution
Now we have 655 mL of solution.
32.0 % V/V means 32 mL of Isopropyl alcohol dissolved in 100 mL of solution.
So,
32 mL Isopropyl alcohol ------------------- 100 mL Solution
x ------------------- 655 mL Solution
x represents the volume of Isopropyl alcohol in 655 mL of solution
[tex]x\text{ = }\frac{655\text{ mL solution x 32 mL }Isopropylalcohol\text{ }}{100\text{ mL solution}}=\text{ 209.6 mL of }Isopropylalcohol[/tex]Now
Alcohol: 209.6 mL
Water: 655 mL (solution = Alcohol + Water) - 209.6 mL (alcohol) = 445.4 mL of water
Please select the correct answer for each question!
Answers
Answer:1=X
2=Y
3=X
Explanation:
General nucleus and electron attraction explained
Answers
General nucleus and electron attraction explained
We can say this:
The net positive charge from the nucleus (remember that protons are in the nucleus) that an electron can feel attraction from, is the effective nuclear charge. The core electrons (electrons are on shells around the nucleus) are said to shield the valence electrons from the full attractive forces of the pro
If 42.6 grams of Al reacts completely with O2 and 57.8 grams of Al2O3 produced, what is the % yield of Al2O3for the reaction? 4Al + 3O2 --------> 2Al2O3Group of answer choices22.3%65.9%55.7%75.2%71.8%
Answers
Step 1
Data provided:
The reaction: 4Al + 3O2 => 2Al2O3 (balanced)
The limiting reactant Al (it is said that Al reacts completely)
Mass of Al = 42.6 g
The actual yield = 57.8 g Al2O3
-----------------------
Step 2
Data needed: the molar mass of Al (26.9 g/mol) and Al2O3 (101.9 g/mol)
The theoretical yield:
4 x 26.9 g Al ------- 2 x 101.9 g Al2O3
42.6 g Al ------- X = 80.7 g Al2O3 = theoretical yield
---------------------
Step 3
% yield = (actual yield/theoretical yield) x 100 = (57.8 g/80.7 g) x 100 = 71.6 %
(this is the closest value for one of the options, 71.8 %)
Answer: 71.8 %
What is the specific heat capacity of methanol (C) if it takes 7,490 joules of energy to heat 50.0 grams from 10.0 0C to 80.0 0C?
Answers
The formula for calculating the amount of heat energy absorbed is given as:
[tex]Q=mc\triangle t[/tex]Given the following
• mass m = 50.0g
,• Heat energy Q = 7490 Joules
,• change in temperature = 80 -10= 70 degrees celsius
Substitute
[tex]\begin{gathered} 7490=50\times c\times70 \\ c=\frac{7490}{3500} \\ c=2.14J\text{/}g^oC \end{gathered}[/tex]Hence the specific heat capacity of methanol is 2.14J/gC
I keep getting 37.5 KPA but apparently that isn't the answer can anyone help?
Answers
ANSWER
The pressure of the gas is 281.25 mmHg
EXPLANATION
Given that;
The initial pressure of the gas is 75 mmHg
The intial temperature of the gas is 80K
The final temperature of the gas is 27 degrees Celcius
Follow the steps below to find the final pressure of the gas
Step 1; Convert the final temperature to degrees kelvin
[tex]\text{ T K = t}\degree C\text{ + 273.15}[/tex][tex]\begin{gathered} \text{ T K = 27 + 273.15} \\ \text{ T K = 300.15K} \end{gathered}[/tex]Step 2; Apply the Gay Lussac's law
[tex]\text{ }\frac{\text{ P1}}{\text{ T1}}\text{ = }\frac{\text{ P2}}{\text{ T2}}[/tex][tex]\begin{gathered} \text{ }\frac{\text{ 75}}{\text{ 80}}\text{ = }\frac{\text{ P2}}{\text{ 300.15}} \\ \text{ cross multiply} \\ \text{ 75 }\times\text{ 300.15 = P2 }\times\text{ 80} \\ 22511.25\text{ = 80 P2} \\ \text{ Divide both sides by 80} \\ \text{ P2 = }\frac{\text{ 22511.25}}{\text{ 80}} \\ \text{ P2 = 281.25mmHg} \end{gathered}[/tex]Read each question carefully and choose the letter of the best answer.
Answers
This element is nonmetal and appears in the periodic table.
The number that represents the position of the elements is called, the atomic number, it also represents the number of protons = numbers of electrons an atom has.
Answer: D. the atomic number
What does the range of a dataset tell us?
A. the value that appears most often in a dataset
B. the difference between the accepted and experimental values
C. the central tendency of the values within a dataset
D. the difference between the lowest and highest values
Answers
The range of a data set tells us that the difference between the lowest and highest values (option D).
What is range in statistics?Range in statistics is the length of the smallest interval which contains all the data in a sample i.e. the difference between the largest and smallest observations in the sample.
The range of a data set is a way to measure the central tendency of a data. It is the largest measure or central tendency.
Range describes how well the central tendency represents the data. If the range of a data is large, the central tendency is not as representative of the data as it would be if the range was small.
Learn more about range at: https://brainly.com/question/20607770
#SPJ1
11.A substance that is corrosive and that conducts electricity in water can be classified as which of the following?Select one:a. An acid only.b. A base only.c. Either an acid or a base.d. Neither an acid or a base.
Answers
Answer
acids and bases are both corrosive furthermore both will conduct electricity depending on the strength of the acid or base
C is the correct option
Calculate the hardness of a water sample which is 35.1 ppm Mg2+ and 65.8 ppm Ca2+.
Answers
The hardness of water sample with 35.1 ppm Mg²⁺ and 65.8 ppm Ca²⁺ is 6.215 ppm is 100.9 ppm
Hardness of water is a parameter which is defined by the amount of dissolved minerals in the water like calcium and magnesium. Thus, hard water would have high amount of dissolved minerals in the water. They are sweeter in taste and are good for bones and teeth because of the mineral content
Total hardness of water = Degree of hardness of Mg ²⁺ + Degree of hardness of Ca ²⁺
Degree of hardness Mg ²⁺ = 35.1 ppm
Degree of hardness of Ca ²⁺ = 65.8 ppm
Total hardness = 35.1 + 65.8 = 100.9 ppm
To know more about Hardness of water
https://brainly.com/question/20936443
#SPJ1
You randomly fina a brick labeled platinum! You get really excited because you look it up and find that platinum is worth $31.86 per gram.then density of platinum is 21.45 g/mL. You measure the volume and it’s 150 cm3 or 150.0mL. How much should the brick weigh if it’s is really platinum? And how much money would the brick be work
Answers
Step 1
Density is defined as:
D = mass of the platinum brick/volume of the platinum brick
D = m/V
------------------
Step 2
Information provided:
D = 21.45 g/mL
V = 150.0 mL
m = unknown
Price per grams of Pt = 31.86 $/g of Pt
Symbol for platinum = Pt (please, look at the periodic table)
-----------------
Step 3
How much should the brick weigh if it’s is really platinum?
From D = m/V
=> D x V = m
=> 21.45 g/mL x 150.0 mL = 3218 g approx.
------
And how much money?
3218 g of Pt x 31.86 $/g of Pt = $99,660 approx.
Answer:
3218 g
$99,660
A sample consists of 75.46% carbon, 4.43% hydrogen, and 20.10% oxygen by mass. Its molar mass is about 318 g/mol.
1.) Determine the empirical formula of the sample.
2.) Determine the molecular formula of the sample.
Answers
The molecular formula is C20H12O4
Suppose you have an Avogadro’s number of potassium. How many grams does this represent?
Answers
According to the given statement one mole of potassium has a mass of 39.1 g.
Briefing:Each element's atomic weight is listed in a periodic table along with how many grams (or an Avogadro number) are needed to make one mole of atoms. Since potassium has an atomic weight of 39.0983 g/mole, a mole of potassium weighs 39.1 g.
What is Avogadro's number explain?Avogadro's number, which is equal to 6.02214076 * 10²³, is the quantity of units in one mole of any material (defined as its molecular weight in grams). Depending on the substance and the nature of the reaction, the units could be electrons, atoms, ions, or molecules.
To know more about Avogadro number visit:
https://brainly.com/question/11907018
#SPJ13
Perform the followingmathematical operation, andreport the answer to thecorrect number of significantfigures.
Answers
To find out how many significant numbers there are in a number we can rely on a few rules:
1. Non-zero digits are always significant
2. Any zeros between non-zero digits are significant
3. Final zeros or trailing zeros are significant if they come after the non-zero numbers in a decimal number situation
Example: .500 and .632000
In this question, we have:
0.0016/0.849 = 0.0019, this is the correct value with the correct number of significant figures
Which compound results from covalent bonding? AgF K2S NaCl CO2
Answers
Answer:CO2
Explanation:
A trick that is quick and easy to use when telling covalent bonds from ionic bonds is to look at the atoms that are bonding. If there is a metal involved, then it is ionic bonding (hence AgF, NaCl. and K2S are not covalent bonding as Ag, Na, and K are all metals.
But to be more specific, ionic bonding happens when two ionic or polar atoms (atoms with non-neutral charges,) come together to balance out their charges (as negative charges will be attracted to positive charges and vice versa); the fancy term for this is electrostatic forces. They (usually) give up electrons to another atom, but do not share electrons. Covalent bonding happens when atoms actually share electrons and have their atomic orbitals overlap, meaning that the electrons actually travel through the surrounding volume of both atoms. Ionic bonded atoms will also separate into ions when in a solvent.
Match each colored row or group on the table to the family of elements it represents.Green (2nd column)Red (1st column)Purple (two rows below the rest of table)Blue (3rd to 12th columns)?Alkali metals?Alkaline earth metals?Transition metals?Lanthanides and actinides
Answers
Green column - Alkaline earth metals
Red column - Alkali metals
Purple column - Lanthanides and actinides
Blue column - Transition metals
Explanations:
The red block are made up of group 1 elements and these elements are known as Alkali metals.
The green column are the group 2 elements in the periodic table and are known as the Alkali earth metals.
For the blue columns, they are element from group 3 to 10 and they are known as the transition metals.
For the purple column, they are Lanthanides and actinides
Humans have been smelting ore for thousands of years. Ancient humans smelted ore to make all but which of the following
Answers
Answer
wiring
Explanation
Ancient humans did make jewelry, weapons and tools by smoltering ore, however, they did not make wires, hence there was no electricity in many mnay years ago.
Select the structure that correspondsto the molecule name:ethylmethylamineA.NHB. CH3CH₂NHCH3C. both
Answers
Ethylmethylamine is a secondary amine, that has one ethyl group attached to the amine group and one methyl group, which is a also attached to the amine group.
It is represented by:
Nevertheless it can be represented by CH3CH2NHCH3.
It means that the correct answer is c. Both.
What is true about the atoms at the start of a chemical reaction compare to the atoms at the end of a net reaction?
Answers
The question requires us to comment on what happens to the atoms in a chemical reaction considering the Law of Conservation of Mass.
The Law of Conservation of Mass states that mass is neither created nor destroyed in a chemical reaction. In other words, although the atoms in the reactants rearrange in order to form the products, the mass of both products and reactants must be the same.
Therefore, we can say that, considering the Law of Conservation of Mass, the atoms at the start of a chemical reaction are rearranged compared to the atoms at the end of the reaction, but the mass of reactants and products do not change.
What is a molecular equation and what is this question asking please help?
Answers
In this question, we have a reaction between silver nitrate and potassium chloride, which will have the following chemical formula and also the following product:
AgNO3 (aq) + KCl (aq) --> AgCl (s) + KNO3 (aq)
Now we need to identify the ions that are components of the precipitate that was formed in this reaction
A precipitate is a product of a reaction that is not soluble in water in the conditions given in the reaction, therefore the precipitate will be a solid product of the reaction. The solid of the reaction is AgCl, therefore this is the precipitate
Now the ions that compose AgCl are Ag+ and Cl-, the answer will be the 3rd option
Name the functional group in thefollowing molecule:NH2A. aldehydeB. amineC. amideD. thiol
Answers
The functional group of the given molecule is located at one of the extremes of it, it is:
This functional group is a carbonyl derived group known as amide. We can recognized because of the carbon that has a double bond with the oxygen and at the same time has a simple bond with nitrogen.
It means that the correct answer is amide.
8) Identify which is the proton acceptor (base) in this following reaction:
HCI + H20 -> CH + H30+
O HCI
O H20
O H30+
O cl
Answers
The proton acceptor (base) in this following reaction is H₃0⁺
Here given reaction is
HCI + H₂O → CH + H₃O⁺
A proton is a subatomic particle found in the nucleus of every atom so in that reaction H₃0⁺ is the proton acceptor because protonic acid is the proton donor H⁺ so H₃0⁺ must be bronsted acid and OH⁻ because it accept the proton is a therefore a bronsted base and a lewis acid is by definition an electron pair acceptor and a lewis base is an electron pair donor
Know more about acceptor
https://brainly.com/question/15231601
#SPJ1
Calculate the volume of 0.875 mol/dm3 NaOH solution needed to make up a 100.0 ml of a diluted solution with a concentration of 0.235 mol/dm3 NaOH.
Answers
The volume of 0.875 mol/dm³ NaOH solution needed to make up a 100.0 ml of a diluted solution with a concentration of 0.235 mol/dm³ NaOH is 73.15 mL.
Given that :
molarity M1 = 0.875 mol/ dm³
M2 = 0.235 mol/ dm³
volume V2 = 100mL
using the formula , we get
M1 V1 = M2 V2
substituting the values in the formula we get :
0.875 × V1 = 0.235 × 100 mL
V1 = 26.857 mL
the volume added = V2 - V1 = 100 mL - 26.85 = 73.15
Thus, The volume of 0.875 mol/dm³ NaOH solution needed to make up a 100.0 ml of a diluted solution with a concentration of 0.235 mol/dm³ NaOH is 73.15 mL.
To learn more about concentration here
https://brainly.com/question/19708372
#SPJ1
If the volume is 15 and the mass of water is 14.9 what is the density
Answers
Answer:
0.9933
(Text Text Text)
What is the concentration of A pill weighing 325mg containing 22mg Mg
Answers
Step 1 - Understanding percentual concentration
To express the concentration of a substance as a percentual concentration, we just need to divide its mass by the total mass:
[tex]concentration\text{ in \% = }\frac{m_{substance}}{m_{total}}\times100[/tex]Step 2 - Calculating the concentration in the pill
We want to calculate the concentration of Mg in the pill. Therefore, we'll divide its mass by the total mass of the pill:
[tex]concentration\text{ of Mg =}\frac{22mg}{325mg}\times100=6.8\text{ \%}[/tex]Answer: the concentration of Mg in the pill is 6.8%