PLEASE ANSWER 50 POINTS!!!!!
How many grams of NH3 form when 22g H2 react completely?
3H2 + N2 ---> 2NH3
H2: 2 g/mol NH3: 17 g/mol
22g H2 ----> gNH3
Answers
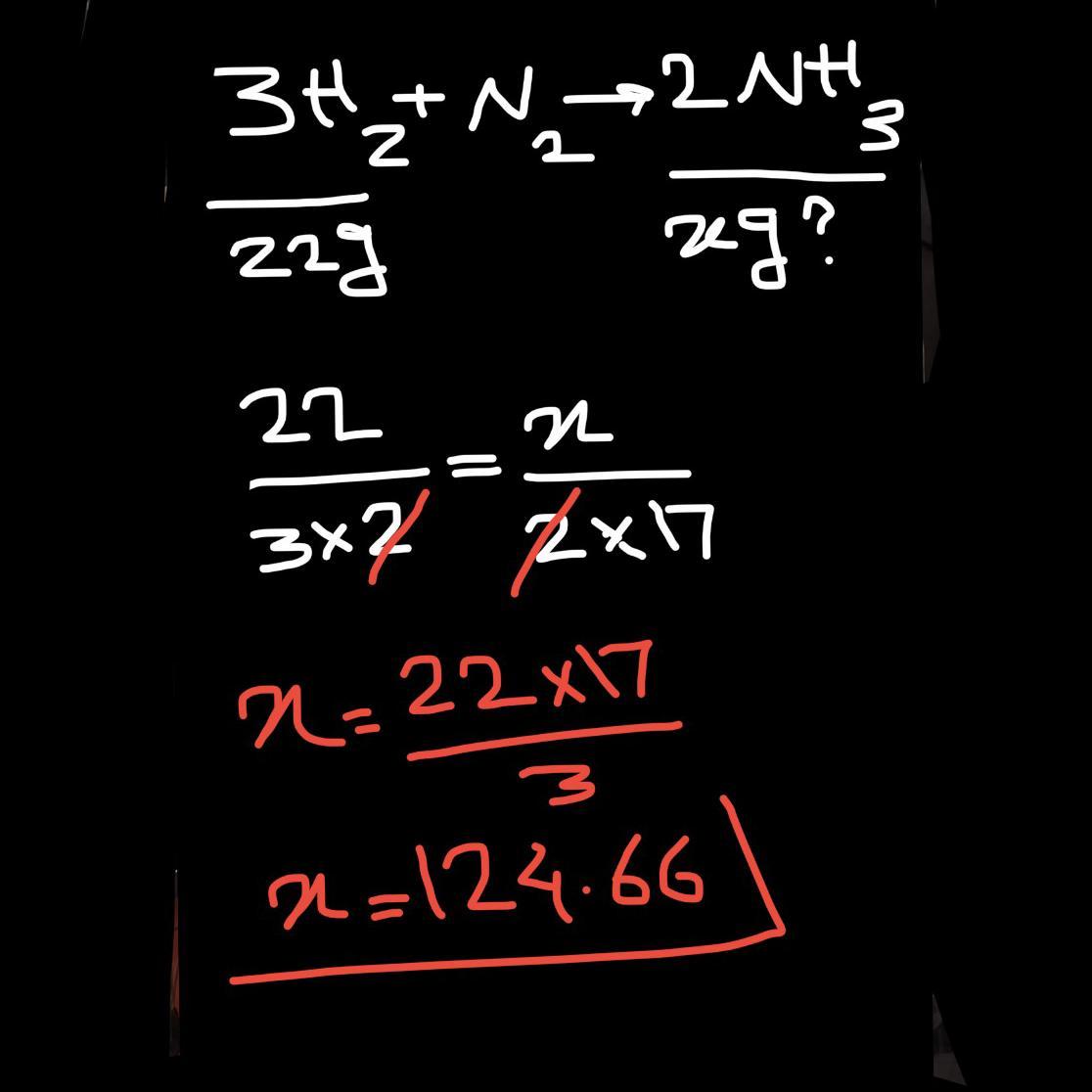
Answer:
mass of NH₃ formed when 22g of H₂ react completely = 124.67 grams
Explanation:
3H₂ + N₂ → 2NH₃
What is stoichiometryThe ratio of coefficients of reactants and products in the above reaction equation (3 : 1 : 2), is known as the stoichiometry of the reaction.
A stoichiometric amount of a reagent is the the optimum amount or ratio where, assuming that the reaction proceeds to completion, all of the reagent is consumed, there is no deficiency of the reagent, and there is no excess of the reagent. Thus if the stoichiometry of a reaction is known, as well as the mass of one of the substances, then it is possible to calculate the mass of any of the other substances.
What is a mole?The mole is a unit of amount of substance established by the International System of Units, to make expressing amounts of reactant or product in a reaction more convenient. As defined by Avogadro's Constant, a mole is 6.022×10²³ amounts of something. The mole is used in stoichiometric calculations, instead of the mass.
Converting between mass and molesTo convert from mass to moles, we need to divide the mass present in grams, by the molar mass of the substance (the sum of the molar masses of the individual elements comprising the compound), in g/mol, to get the moles. This can be represented by the formula: n = m/M, where n = number of moles, m = mass, M = molar mass.
So if we have 22 g of H₂ gas, which reacts completely, and therefore is a stoichiometric amount, then converting this to moles:
n(H₂) = m/M = 22/2 = 11 mol.
Using our stoichiometry, we can see that the ratio of H₂ to NH₃ = 3 : 2.
Therefore, for every 3 moles of H₂ used, we produce 2 moles of NH₃.
n(NH₃) = 2/3 × n(H₂) = 2/3 × 11 = 7.333 mol.
Finally, converting moles back to mass we get:
m(NH₃) = n×M = 7.333×17 = 124.67 grams
∴ mass of NH₃ formed when 22g of H₂ react completely = 124.67 grams
Related Questions
why do you think the procedure directed you to perform each of the tests on a sample of distilled water in addition to the carbohydrate samples?
Answers
The purpose of performing the tests on a sample of distilled water is to establish a baseline or control in order to compare the results of the tests on the carbohydrate samples.
The results of the tests on the distilled water should indicate the presence of only a few components such as hydrogen and oxygen and no other compounds. This allows scientists to compare the results of the tests on the carbohydrate samples and easily identify any compounds that are present in the sample that are not present in the control.
This way, the presence of any contaminants can be detected and the results of the tests can be accurately interpreted.
Know more about distilled water here.
https://brainly.com/question/23802525#
#SPJ11
Precautions List precautions and explain why they were taken:
when adding water to the rock salt.
during the filtration stage.
during (i) evaporation to dryness and (ii) crystallisation.
Answers
Precautions when adding water to rock salt: Add water slowly and carefully to avoid splashing ; Precautions during filtration stage: Use filter paper that fits the funnel properly ; Precautions during (i) evaporation to dryness and (ii) crystallization: Avoid overheating solution during evaporation and stirring the solution.
What is meant by evaporation?Physical process by which a liquid substance is transformed into gaseous state is called evaporation.
Precautions and their explanations:
Precautions when adding water to rock salt:
Add water slowly and carefully to avoid splashing or spilling.
Use a stirring rod to dissolve salt crystals completely.
Explanation: Rock salt can be quite reactive with water, and adding too much water too quickly can cause the solution to boil or splatter. Using a stirring rod helps to dissolve salt crystals completely without creating too much agitation.
Precautions during filtration stage:
Use a filter paper that fits the funnel properly and fold it properly.
Avoid touching filter paper with your fingers.
Explanation: The filter paper needs to fit the funnel properly to ensure that all of the liquid is filtered properly.
Precautions during (i) evaporation to dryness and (ii) crystallization:
Avoid overheating solution during evaporation and stirring the solution.
Use a clean glass rod to encourage crystallization and avoid scratching the walls of the container.
Explanation: Overheating the solution can cause the salt to decompose or change its chemical properties. Stirring the solution can also lead to the formation of smaller crystals.
To know more about evaporation, refer
https://brainly.com/question/9339710
#SPJ1
What types of pros and cons might you need to consider when evaluating different energy sources, such as oil, gas, solar, and wind?
Answers
Despite being simpler to store and transport than other fossil fuels and renewables, natural gas has one significant storage drawback. Its volume is four times more than that of petrol. As a result, natural gas storage is substantially more expensive since more storage area is required.
How many solar panels are required to power a home?To fully offset power expenditures with solar, a typical home need between 17 and 21 solar panels. The amount of solar panels you require is determined by a few main criteria, including your geographic location and the specs of individual panels.
Renewable energy sources provide the majority of their energy at specific times of the day. Its electrical generation does not correspond with peak demand hours.
learn more about solar energy
https://brainly.com/question/17711999
#SPJ1
A sample of oxygen (O2) gas occupies a volume of 251 mL at 735 torr of pressure. Calculate the volume the oxygen will occupy if the pressure changes to 825 torr.
Answers
The volume the oxygen will occupy if the pressure changes to 825 torr is 223.62 mL.
How to calculate volume?The volume of a gas with a changing pressure can be calculated in accordance to Boyle's law as follows;
P₁V₁ = P₂V₂
Where;
P₁ and V₁ = initial pressure and volumeP₂ and V₂ = final pressure and volumeAccording to this question, a sample of oxygen gas occupies a volume of 251 mL at 735 torr of pressure. If the pressure changes to 825 torr, the new volume can be calculated as follows:
251 × 735 = V × 825
V = 184,485 ÷ 825
V = 223.62 mL
Learn more about volume at: https://brainly.com/question/24189159
#SPJ1
2 NO(g)+Cl2(g)⇌2 NOCl(g) Kc=2000
A mixture of NO(g) and Cl
2
(g) is placed in a previously evacuated container and allowed to reach equilibrium according to the chemical equation shown above When the system reaches equilibrium, the reactants and products have the concentrations listed in the following table:
Species Concentration (M)
NO(g) 0.050
C12(g) 0.050
NOCl(g) 0.50
Which of the following is true if the volume of the container is decreased by one-half?
A. Q = 100, and the reaction will proceed toward reactants.
B. Q = 100, and the reaction will proceed toward products.
C. Q = 1000, and the reaction will proceed toward reactants.
D. Q = 1000, and the reaction will proceed toward products.
Answers
Neither A, B, C nor D. The equilibrium position will not be affected by the change in volume.
To determine how the equilibrium of the reaction 2 NO(g) + Cl₂(g) ⇌ 2 NOCl(g) will shift if the volume of the container is decreased by one-half, we first need to calculate the reaction quotient Q.
The balanced chemical equation for the reaction is:
2 NO(g) + Cl₂(g) ⇌ 2 NOCl(g)
At equilibrium, the concentrations of the species are:
[NO] = 0.050 M
[Cl2] = 0.050 M
[NOCl] = 0.50 M
Using these values, we can calculate the value of the reaction quotient Q:
Q [tex]= [NOCl]^2 / ([NO]^2[Cl2])[/tex]= [tex](0.50)^2 / ((0.050)^2 x 0.050)[/tex] = 1000
Now we compare the value of Q to the equilibrium constant Kc:
Kc =[tex][NOCl]^2 / ([NO]^2[Cl2])[/tex] = 2000
Since Q < Kc, we can conclude that the reaction has not yet reached equilibrium and that the forward reaction will proceed to reach equilibrium.
When the volume of the container is decreased by one-half, the concentration of all species will increase due to the decrease in volume. According to Le Chatelier's principle, the reaction will shift in the direction that reduces the total number of moles of gas.
In this case, the reaction produces two moles of gas on the left-hand side and two moles of gas on the right-hand side, so the total number of moles of gas does not change. Therefore, the volume change will not have an effect on the equilibrium position.
Learn more about equilibrium here:
https://brainly.com/question/30807709
v
#SPJ11
The correct answer is: C. Q = 1000, and the reaction will proceed toward reactants.
How to determine the reactions at equilibrium?
To determine which statement is true if the volume of the container is decreased by one-half, we need to calculate the reaction quotient (Q) for the new conditions.
When the volume is decreased by half, the concentrations of all species will double:
NO(g): 0.050 * 2 = 0.100 M
Cl2(g): 0.050 * 2 = 0.100 M
NOCl(g): 0.50 * 2 = 1.00 M
Now, calculate Q using the new concentrations:
Q = [NOCl]^2 / ([NO]^2 * [Cl2])
Q = (1.00)^2 / ((0.100)^2 * (0.100))
Q = 1 / 0.001
Q = 1000
So, Q = 1000. Now, compare Q to Kc:
Q > Kc, meaning the reaction will proceed toward the reactants to reach equilibrium.
To know more about Reaction Quotient:
https://brainly.com/question/24202150
#SPJ11
one kg of butane (c4h10) is burned with 25 kg of air that is at 30c and 90kpa. assuming the combustion is complete, determine the percentage of theoretical air used?
Answers
The percentage of theoretical air used is approximately 190.3%.
To determine the percentage of theoretical air used in the combustion of 1 kg of butane (C4H10), we need to calculate the amount of air required for complete combustion and compare it to the actual amount of air used.
The balanced chemical equation for the combustion of butane is:
[tex]C_4H_{10} + 13/2 O_2 - > 4 CO_2 + 5 H_2O[/tex]
This means that for every mole of butane that is burned, 13/2 moles of oxygen are required. The molar mass of butane is 58.12 g/mol, so 1 kg of butane is equivalent to 17.20 moles.
Therefore, the amount of oxygen required for complete combustion of 1 kg of butane is:
(13/2) mol O_2/mol butane x 17.20 mol butane = 111.4 mol O_2
Next, we need to calculate the amount of air required for complete combustion. Air is approximately 21% oxygen and 79% nitrogen by volume. Therefore, the volume of air required for complete combustion is:
111.4 mol O_2 / (0.21 mol O2/mol air) = 530.5 mol air
Assuming ideal gas behavior, the volume of air at 30°C and 90 kPa can be calculated using the ideal gas law
PV = nRT
where P is the pressure (90 kPa), V is the volume, n is the number of moles of air, R is the gas constant, and T is the temperature in Kelvin (303 K).
V = nRT/P = (530.5 mol x 0.08206 L atm K^-1 mol^-1 x 303 K) / (90 kPa x 101.3 kPa/atm) = 12,425 L
Therefore, the percentage of theoretical air used in the combustion of 1 kg of butane is:
(actual air used / theoretical air required) x 100%
= (25,000 g air / 12,425 L) / (530.5 mol air / 1 kg butane) x 100%
= 190.3
So, the percentage of theoretical air used is approximately 190.3%. This value is greater than 100% because the actual amount of air used is more than the theoretical amount due to the excess nitrogen present in air.
To learn more about : theoretical
https://brainly.com/question/14714924
#SPJ11
For a mechanical change in an isolated system, the mechanical
energy at the beginning equals the mechanical energy at the
end of the process, as long as friction is negligible.
O True
O False
Answers
For a mechanical change in an isolated system, the mechanical energy at the beginning equals the mechanical energy at the end of the process, as long as friction is negligible. This statement is true.
The combination of kinetic energy, meaning energy of motion, with potential energy, meaning energy retained by a system as a result of the arrangement of its components, is known as mechanical energy. A system with solely gravitational forces or one that is otherwise idealized.
For a mechanical change in an isolated system, the mechanical energy at the beginning equals the mechanical energy at the end of the process, as long as friction is negligible. This statement is true.
To know more about mechanical energy, here:
https://brainly.com/question/29509191
#SPJ1
calculate the volume of a stock solution, in liters and to the thousandths place, that has a concentration of 0.400 m koh and is diluted to 3.00 l of 0.130 m koh
Answers
The volume of the stock solution is approximately 0.975 liters, to the thousandths place.
To calculate the volume of the stock solution, you can use the dilution formula:
C₁V₁ = C₂V₂
where:
C₁ = concentration of the stock solution (0.400 M KOH)
V₁ = volume of the stock solution (unknown, in liters)
C₂ = concentration of the diluted solution (0.130 M KOH)
V₂ = volume of the diluted solution (3.00 L)
Rearrange the formula to solve for V1:
V1 = C₂V₂ / C₁
Now, plug in the given values:
V₁ = (0.130 M KOH * 3.00 L) / 0.400 M KOH
V₁ ≈ 0.975 L
know more about stock solution here
https://brainly.com/question/25256765#
#SPJ11
a 16.60 ml portion of 0.0969 m ba(oh)2 was used to titrate 25.0 ml of a weak monoprotic acid solution to the stoichiometric point. what is the molarity of the acid?
Answers
The molarity of the weak monoprotic acid solution is 0.0644 mol/L.
To find the molarity of the acid, we need to use the balanced chemical equation and the stoichiometry of the reaction between the acid and the base. The equation for the reaction is:
HA(aq) + Ba(OH)2(aq) → BaA2(aq) + 2H2O(l)
where HA is the weak monoprotic acid, Ba(OH)2 is the strong base, BaA2 is the barium salt of the acid, and H2O is water.
At the stoichiometric point, the moles of Ba(OH)2 used will be equal to the moles of acid present in the solution. Using the given volume and molarity of Ba(OH)2, we can calculate the moles of Ba(OH)2 used:
moles of Ba(OH)2 = volume × molarity = 16.60 ml × 0.0969 mol/L = 0.00161 mol
Since the acid is a monoprotic acid, the moles of acid present in the solution will be equal to the moles of Ba(OH)2 used. Therefore:
moles of HA = 0.00161 mol
Using the volume of the acid solution (25.0 ml), we can calculate the molarity of the acid:
molarity of HA = moles of HA / volume of HA solution in L
molarity of HA = 0.00161 mol / 0.0250 L
molarity of HA = 0.0644 mol/L
For such more questions on Molarity:
https://brainly.com/question/14469428
#SPJ11
A vinegar solution of unknown concentration was prepared by diluting 10. 00 mL of vinegar to a total volume of 50. 00 mL with deionized water. A 25. 00-mL sample of the diluted vinegar solution required 20. 24 mL of 0. 1073 M NaOH to reach the equivalence point in the titration. Calculate the concentration of acetic acid, CH3COOH, (in M) in the original vinegar solution (i. E. , before dilution)
Answers
The concentration of acetic acid in the original vinegar solution is 0.0435M.
Balanced chemical equation for the reaction between acetic acid (CH₃COOH) and sodium hydroxide (NaOH) is:
CH₃COOH + NaOH → CH₃COONa + H₂O
The number of moles of NaOH used in the titration will be calculated as;
moles NaOH = Molarity × Volume (in L)
moles NaOH = 0.1073 M × 0.02024 L
moles NaOH = 0.002174872
Therefore, the concentration of CH₃COOH in the diluted vinegar solution is;
C₁V₁ = C₂V₂
C₁ × 10.00 mL = C₂ × 50.00 mL
C₁ = (C₂ × 50.00 mL) ÷ 10.00 mL
C₁ = 5 × C₂
where C₁ is the concentration of CH₃COOH in the diluted vinegar solution, and C₂ is the concentration of CH₃COOH in the original vinegar solution.
The number of moles of CH₃COOH in the diluted vinegar solution is;
moles CH₃COOH = C₁ × V₁ (in L)
moles CH₃COOH = (5 × C₂) × 0.01000 L
moles CH₃COOH = 0.05000 × C₂
The concentration of CH₃COOH in the original vinegar solution can be calculated;
moles CH₃COOH in original vinegar = moles CH₃COOH in diluted vinegar
0.05000 × C₂ = 0.002174872
C₂ = 0.002174872 ÷ 0.05000
C₂ = 0.043
To know more about concentration here
https://brainly.com/question/10725862
#SPJ4
What is one way someone could benefit from the non-separation of a colloid mixture? Explain.
Answers
An example are the emulsions used in the food industry.
How someone could benefit from the non-separation of a colloid mixture?One way someone could benefit from the non-separation of a colloid mixture is in the case of emulsions, which are a type of colloid mixture. Emulsions are mixtures of immiscible liquids, such as oil and water, stabilized by an emulsifying agent.
The non-separation of emulsions can be beneficial in various practical applications, such as the food Industry, where emulsions are commonly used in the food industry to create a wide range of products, including salad dressings, mayonnaise, sauces, and margarine. Emulsions provide desirable texture, appearance, and taste properties to these food products, and their non-separation allows for long shelf life and consistent quality.
Learn moer about emulsions at:
https://brainly.com/question/6711819
#SPJ1
phenacetin can be prepared from p-acetamidophenol, which has a molar mass of 151.16 g/mol, and bromoethane, which has a molar mass of 108.97 g/mol. the density of bromoethane is 1.47 g/ml. what is the yield in grams of phenacetin, which has a molar mass of 179.22 g/mol, possible when reacting 0.151 g of p-acetamidophenol with 0.12 ml of bromoethane?
Answers
The theoretical yield of phenacetin is 0.17922 g. However, the actual yield may be lower due to factors such as incomplete reaction, loss during purification, or experimental error.
To calculate the theoretical yield of phenacetin, we need to first determine the limiting reagent. The limiting reagent is the reactant that will be completely consumed in the reaction, thus limiting the amount of product that can be produced.
First, we need to convert the volume of bromoethane given in milliliters to grams, using its density:
0.12 ml x 1.47 g/ml = 0.1764 g bromoethane
Next, we can use the molar masses of p-acetamidophenol and bromoethane to determine the number of moles of each:
moles p-acetamidophenol = 0.151 g / 151.16 g/mol = 0.001 mol
moles bromoethane = 0.1764 g / 108.97 g/mol = 0.00162 mol
Since the reaction requires a 1:1 molar ratio of p-acetamidophenol to bromoethane, and the number of moles of p-acetamidophenol is smaller than the number of moles of bromoethane, p-acetamidophenol is the limiting reagent.
The theoretical yield of phenacetin can be calculated using the molar mass of phenacetin and the number of moles of p-acetamidophenol:
moles phenacetin = 0.001 mol p-acetamidophenol
mass phenacetin = 0.001 mol x 179.22 g/mol = 0.17922 g
For such more questions on Phenacetin:
https://brainly.com/question/29460577
#SPJ11
rade 11 Text Books Exercise 5.4 Answer the following questions: 1. 5.0 mole of ammonia were introduced into a 5.0 L reaction chamber in which it is partially decomposed at high temperatures. CHEMISTRY GRADE 11 267 2NH₂(g) 3H₂(g) + N₂(g) At equilibrium at a particular temperature, 80.0% of the ammonia had reacted. Calculate K for the reaction.
Answers
At the given temperature, the equilibrium constant K for the reaction is 0.5625 mol/L.
How to determine equilibrium constant?The balanced chemical equation for the reaction is:
2NH₃(g) ⇌ 3H₂(g) + N₂(g)
The equilibrium expression for the reaction is:
K = [H₂]³[N₂] / [NH₃]²
Given that 5.0 moles of NH₃ were introduced into a 5.0 L reaction chamber, the initial concentration of NH₃ is:
[NH₃]₀ = 5.0 mol / 5.0 L = 1.0 mol/L
At equilibrium, 80.0% of the NH₃ had reacted, which means that 20.0% of NH₃ remains. Therefore, the equilibrium concentration of NH₃ is:
[NH₃] = 0.20 x 1.0 mol/L = 0.2 mol/L
The equilibrium concentrations of H₂ and N₂ can be calculated from the balanced equation:
[H₂] = (3/2) x [NH₃] = 0.3 mol/L
[N₂] = [NH₃] / 2 = 0.1 mol/L
Substituting these values into the equilibrium expression gives:
K = [H₂]³[N₂] / [NH₃]²
K = (0.3 mol/L)³ x (0.1 mol/L) / (0.2 mol/L)²
K = 0.5625 mol/L
Therefore, the equilibrium constant K for the reaction at the given temperature is 0.5625 mol/L.
Find out more on equilibrium constant here: https://brainly.com/question/19340344
#SPJ1
6. from the lab on solutions, what is the criterion for determining whether or not a solution is a conductor of electricity?
Answers
In the lab on solutions, the criterion for determining whether or not a solution is a conductor of electricity is the presence of free-moving ions within the solution. When a substance dissolves in water and releases ions, it allows the flow of electric current, making it a conductor of electricity.
The criterion for determining whether or not a solution is a conductor of electricity is whether or not it contains ions that are able to move freely and carry an electric charge. A solution that contains ions is considered a conductor of electricity, while a solution that does not contain ions is considered a non-conductor or insulator of electricity.
Learn more about conductors of electricity at https://brainly.com/question/3447552
#SPJ11
The criterion for determining whether or not a solution is a conductor of electricity is whether or not it contains ions that can carry an electric charge.
If the solution contains ions, it can act as a conductor of electricity. If it does not contain ions, it will not conduct electricity.
Use the following criterion:
A solution is considered a conductor of electricity if it contains ions that are free to move. These ions enable the flow of electrical current through the solution. Typically, this occurs when a solution has dissolved salts, acids, or bases, as they dissociate into ions when dissolved in a solvent like water. To test the conductivity of a solution, you can use a simple conductivity meter or a circuit with a light bulb, and observe if the light bulb lights up or if the meter shows any electrical current flow. If it does, the solution is a conductor of electricity.
Learn more about conductor here:
https://brainly.com/question/15320131
#SPJ11
A buffer solution contains 0.10 mol of acetic acid and 0.14 mol of sodium acetate in 1.00 L. What is the pH of the buffer after the addition of 0.03 mol of KOH?
Answers
The pH of the buffer after the addition of 0.03 mol of KOH is 5.04.
To answer this question, we need to use the Henderson-Hasselbalch equation, which relates the pH of a buffer solution to the concentration of the acid and its conjugate base:
pH = pKa + log([A-]/[HA])
where pKa is the dissociation constant of the acid, [A-] is the concentration of the conjugate base (in this case, sodium acetate), and [HA] is the concentration of the acid (acetic acid).
First, we need to calculate the initial concentrations of acetic acid and sodium acetate:
[HA] = 0.10 mol/L
[A-] = 0.14 mol/L
Next, we need to calculate the new concentrations of acetic acid and sodium acetate after the addition of 0.03 mol of KOH. Since KOH is a strong base, it will react completely with the acetic acid to form acetate ion:
CH3COOH + KOH -> CH3COO- + H2O
The amount of acetic acid that reacts with KOH is:
0.03 mol KOH / 1 L = 0.03 M
Since acetic acid and KOH react in a 1:1 ratio, the concentration of acetic acid is now:
[HA] = 0.10 mol/L - 0.03 mol/L = 0.07 mol/L
The amount of acetate ion that is formed is also 0.03 mol/L, since acetic acid and acetate ion are in equilibrium:
CH3COOH <--> CH3COO- + H+
Since the buffer initially contained 0.14 mol/L of sodium acetate, the new concentration of acetate ion is:
[A-] = 0.14 mol/L + 0.03 mol/L = 0.17 mol/L
Now we can calculate the pH of the buffer using the Henderson-Hasselbalch equation:
pH = 4.76 + log(0.17/0.07) = 5.04
To learn more about Henderson-Hasselbalch equation click here
brainly.com/question/13423434
#SPJ11
which of the following is true about the absorption and metabolism of alcohol? alcohol is metabolized by most tissue and organs in the body. the majority of alcohol is absorbed in the stomach. men and women do not metabolize alcohol at significantly different rates. acetaldehyde produced during alcohol metabolism is highly toxic.
Answers
The statement "acetaldehyde produced during alcohol metabolism is highly toxic" is true about absorption and metabolism of alcohol. Option 4 is correct.
Acetaldehyde is a byproduct of alcohol metabolism, and it is a toxic substance that can cause various symptoms such as facial flushing, nausea, and headache. Acetaldehyde is rapidly converted to acetate by the enzyme aldehyde dehydrogenase, which is then metabolized further to carbon dioxide and water.
However, if alcohol is consumed at a high rate, the liver may not be able to metabolize all of the acetaldehyde, leading to a buildup of this toxic substance in the body. This can result in more severe symptoms such as vomiting, rapid heartbeat, and difficulty breathing. Therefore, it is important to consume alcohol in moderation and allow enough time for the liver to metabolize the alcohol and its byproducts. Hence Option 4 is correct.
To learn more about absorption and metabolism of alcohol, here
https://brainly.com/question/14310421
#SPJ4
what is the effect on the half-potential at 35 c when the ph of the solution is decreased by one unit
Answers
When the pH of a solution is decreased by one unit, the concentration of H+ ions increases This, in turn, can affect the half-potential of the solution. In acidic solutions,
The half-potential of a solution is a measure of its tendency to either gain or lose electrons. the concentration of H+ ions is high, leading to a decrease in the half-potential. When the pH of a solution is decreased by one unit, the half-potential of the solution will likely decrease if the solution is acidic.
Conversely, in alkaline solutions, the concentration of OH- ions is high, leading to an increase in the half-potential. The effect of pH on the half-potential is significant in electrochemical reactions,
as it can influence the overall reaction rate and the efficiency of the reaction. It is important to carefully monitor the pH of a solution in electrochemical experiments to ensure accurate results.
To learn more about : half-potential
https://brainly.com/question/18917494
#SPJ11
What is the mass of ether(0. 71) which can be put into a beaker holding 130ml
Answers
The mass of ether that can be put into a 130 mL beaker is approximately 92.3 grams.
How to find the mass of the etherTo calculate the mass of ether that can be put into a 130 mL beaker, we need to know the density of ether.
The density of ether varies depending on the specific type of ether, but assuming you are referring to diethyl ether, the density is approximately 0.71 g/mL.
Using the density and the volume of the beaker, we can calculate the maximum mass of ether that can be put into the beaker as follows:
Mass of ether = Density x Volume
Mass of ether = 0.71 g/mL x 130 mL
Mass of ether = 92.3 grams
Therefore, the maximum mass of diethyl ether that can be put into a 130 mL beaker is approximately 92.3 grams.
Learn more about density at
https://brainly.com/question/26364788
#SPJ1
a solution is 0.0300m in both cro42- and so42-. slowly, pb(no3)2 is added to this solution. what is the concentration of cro42- that remains in solution when pbso4 first begins to precipitate? ksp of pbcro4
Answers
The concentration of [tex](CrO_4)^{2-[/tex]that remains in solution when [tex]PbSO_4[/tex] first begins to precipitate is zero.
When [tex]PbSO_4[/tex] is added to the solution containing 0.0300 M of both [tex](CrO_4)^{2-[/tex]and [tex](SO_4)^{2-[/tex], a precipitation reaction occurs where [tex]PbCrO_4[/tex] (lead chromate) and PbSO4 (lead sulfate) are formed.
The Ksp (solubility product constant) of [tex]PbCrO_4[/tex] is 1.8 x 10^-14 at 25°C. As more [tex]Pb(NO_3)^2[/tex]is added, the concentration of Pb2+ increases until it reaches a point where the Ksp of[tex]PbCrO_4[/tex] is exceeded and precipitation occurs.
At this point, all of the [tex](CrO_4)^{2-[/tex] ions have reacted with [tex]Pb^{2+[/tex] to form [tex]PbCrO_4[/tex], and the concentration of [tex](CrO_4)^{2-[/tex] in solution is zero. The precipitation of [tex]PbCrO_4[/tex] will continue until all of the [tex]Pb^{2+[/tex] ions have reacted with [tex](CrO_4)^{2-[/tex] ions, at which point [tex]PbSO_4[/tex] will begin to precipitate.
To learn more about : concentration
https://brainly.com/question/28564792
#SPJ11
4. describe the relationship between the metal and water in terms of which is exothermic and which is endothermic.
Answers
The relationship between metal and water is highly dependent on the specific metal and the conditions under which they react with water. In general, however, the reaction between metals and water can be either exothermic or endothermic.
For highly reactive metals like sodium or potassium, the reaction with water is highly exothermic, meaning that it releases a large amount of heat. This is because these metals readily react with water to produce hydrogen gas and a highly alkaline solution of metal hydroxide. For example, the reaction between sodium and water can be represented as:
2 Na(s) + 2 H2O(l) → 2 NaOH(aq) + H2(g) + heat
On the other hand, for less reactive metals like copper or silver, the reaction with water is usually endothermic, meaning that it absorbs heat from the surroundings. In these cases, the reaction occurs very slowly or not at all, and the metal may simply become coated with a layer of metal oxide or hydroxide. For example, the reaction between copper and water is relatively slow and can be represented as:
2 Cu(s) + O2(g) + 2 H2O(l) → 2 Cu(OH)2(s)
In summary, the relationship between metal and water in terms of exothermic and endothermic reactions is highly dependent on the specific metal and the conditions under which they react. Highly reactive metals tend to have exothermic reactions with water, while less reactive metals tend to have endothermic reactions or no reaction at all.
what is a possible set of quantum numbers m, l, ml, ms for the electron configuration of cobalt g
Answers
One possible set of quantum numbers for cobalt's electron configuration is:
m = -2, -1, 0, 1, 2, 1, 0
l = 2
ml = -2, -1, 0, 1, 2, 0, 1
ms = +1/2, -1/2, +1/2, -1/2, +1/2, -1/2, +1/2
The electron configuration of cobalt in its ground state is:
1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^7
To determine the possible set of quantum numbers, we need to first fill the orbitals in the order of increasing energy and the Pauli exclusion principle, Hund's rule, and the aufbau principle.
The last electron enters the 3d subshell, which has five orbitals (dxy, dyz, dxz, dx2-y2, and dz2). The possible quantum numbers for the last electron in the 3d subshell are:
ml can have values from -2 to +2, corresponding to the five d orbitals.
l = 2 since d orbitals have an azimuthal quantum number of 2.
ms can have values of +1/2 or -1/2, corresponding to the electron's spin.
Since there are seven electrons in the 3d subshell, we can have up to seven sets of quantum numbers for the seven electrons. One possible set of quantum numbers for cobalt's electron configuration is:
m = -2, -1, 0, 1, 2, 1, 0
l = 2
ml = -2, -1, 0, 1, 2, 0, 1
ms = +1/2, -1/2, +1/2, -1/2, +1/2, -1/2, +1/2
Note that the last three electrons must have opposite spins (Pauli exclusion principle), and each orbital can have at most two electrons (Hund's rule).
Click the below link, to learn more about Electron Configuration of cobalt:
https://brainly.com/question/19863670
#SPJ11
the alkane c7h16 exhibits structural isomerism. in fact, 9 structural isomers have this same formula (but different bond arrangements). one such isomeric structure is:
Answers
Systematic name of this structure is 3-ethylpentane.
Chemical compounds known as isomers have identical chemical formulae but have different properties and atom arrangements inside the molecule. The term "isomer" refers to a substance that exhibits isomerism.
Structural isomers are substances with the same molecular formula but distinct atomic configurations. The way the atoms are attached in this instance is quite different, as seen by the different types of chains that are formed (straight versus branched), the placements of the atoms (such as middle versus end of the parent chain), and the presence of functional groups (e.g., aldehydes versus ketones).
For instance, although sharing the same molecular formula (C3H6O), propanal and propanone have very distinct chemical structures. They are structural isomers as a result.
Isomers of Heptane are:
Heptane (n-heptane)2-Methylhexane (iso-heptane)3-Methylhexane2,2-Dimethylpentane (neo-heptane)2,3-Dimethylpentane2,4-Dimethylpentane3,3-Dimethylpentane3-Ethylpentane2,2,3-TrimethylbutaneTo learn more about isomers, refer:
brainly.com/question/12796779
#SPJ4
The complete question is: The alkane C7H16 exhibits structural isomerism. In fact, 9 structural isomers have this same formula (but different bond arrangements). One such isomeric structure is: What is the correct systematic name for this structure?
according to the ismp, which of the following is appropriate? select one: a. 100000 units b. 0.9% sodium chloride c. .9% sodium chloride d. 1.0 mg
Answers
According to the ISMP, the appropriate option is "0.9% sodium chloride" as it is written in the correct format with the percentage symbol and the correct concentration of sodium chloride.
The other options do not relate to the given terms or are not written in the appropriate format. The option "1.0 mg" is written in the correct format but does not relate to sodium chloride or the given scenario.
According to the ISMP (Institute for Safe Medication Practices), the appropriate option among the given choices is:
b. 0.9% sodium chloride
This option is appropriate because it clearly specifies the concentration of the sodium chloride solution, which is essential for accurate and safe medication administration. The other options (a, c, and d) lack context or contain ambiguous information, which could lead to medication errors or incorrect dosing.
Learn more about sodium chloride here:
https://brainly.com/question/29801408
#SPJ11
According to the ISMP, the appropriate term would be "0.9% sodium chloride".
How to represent concentrations according to ISMP?
This is because the ISMP recommends using a leading zero before a decimal point for concentrations and avoiding the use of ambiguous or error-prone abbreviations, such as option C (.9% sodium chloride) which lacks a leading zero. Option A (100000 units) and option D (1.0 mg) are not relevant to the context of the question. Therefore, the correct format is "0.9%" rather than ".9%" or "1.0 mg".
To know more about ISMP:
https://brainly.com/question/31018598
#SPJ11
a 35.0-ml sample of 0.20 m lioh is titrated with 0.25 m hcl. what is the ph of the solution after 23.0 ml of hcl have been added to the base? group of answer choices 1.26 12.74 12.33 13.03 1.67
Answers
The pH of the solution after 23.0 mL of 0.25 M HCl have been added to the 35.0 mL of 0.20 M LiOH is 12.74.
1. Calculate the initial moles of LiOH and HCl:
LiOH: 35.0 mL * 0.20 mol/L = 7.00 mmol
HCl: 23.0 mL * 0.25 mol/L = 5.75 mmol
2. Determine the limiting reactant and find the moles of unreacted LiOH:
Since HCl is the limiting reactant, subtract its moles from LiOH moles:
7.00 mmol - 5.75 mmol = 1.25 mmol of unreacted LiOH
3. Calculate the new concentration of LiOH in the solution:
Total volume: 35.0 mL + 23.0 mL = 58.0 mL
New concentration: 1.25 mmol / 58.0 mL = 0.02155 mol/L
4. Calculate the pOH of the solution:
pOH = -log10(0.02155) = 1.66
5. Find the pH of the solution:
pH = 14 - pOH = 14 - 1.66 = 12.74
To know more about pH click on below link:
https://brainly.com/question/491373#
#SPJ11
which method would you use to perform these reactions, grignard carboxylation or nitrile hydrolysis?
Answers
Choose the method based on your starting material: Grignard carboxylation for alkyl halide and Nitrile hydrolysis for nitriles
If the desired reactions involve the conversion of a nitrile functional group to a carboxylic acid, then the method that should be used is nitrile hydrolysis. Grignard carboxylation is a different chemical process that involves the addition of a Grignard reagent to a carbonyl group to form a carboxylic acid. Therefore, nitrile hydrolysis would be the appropriate method for the conversion of a nitrile to a carboxylic acid.
Hi! To determine the appropriate method for your reactions, let's briefly discuss each one:
1. Grignard carboxylation: This reaction involves the use of a Grignard reagent (an organomagnesium compound, typically R-MgX) reacting with carbon dioxide (CO2) to produce a carboxylic acid. It's a useful method for preparing carboxylic acids from alkyl halides.
2. Nitrile hydrolysis: This reaction involves the conversion of a nitrile (RC≡N) to a carboxylic acid (RCOOH) by reacting with water in the presence of an acid or a base as a catalyst. This method is suitable for preparing carboxylic acids from nitriles.
If your starting material is a nitrile, the appropriate method to perform the reaction would be nitrile hydrolysis. If your starting material is an alkyl halide, you would use the Grignard carboxylation method.
In summary, choose the method based on your starting material:
- Grignard carboxylation for alkyl halides
- Nitrile hydrolysis for nitriles
Learn more about Grignard carboxylation here:
https://brainly.com/question/9322175
#SPJ11
The process chosen is determined on the starting material and the intended product. Grignard carboxylation is a better procedure if the starting material is an alkyl or aryl halide and the target product is a carboxylic acid. If the starting material is a nitrile and the desired product is a carboxylic acid, nitrile hydrolysis is the procedure to use.
Grignard carboxylation is a useful method for the synthesis of carboxylic acids from alkyl and aryl halides. In this reaction, a Grignard reagent (an organomagnesium compound) is first prepared by reacting an alkyl or aryl halide with magnesium metal.
The resulting Grignard reagent is then reacted with carbon dioxide to form a carboxylate intermediate, which is subsequently hydrolyzed with an acid to produce the carboxylic acid.
Nitrile hydrolysis, on the other hand, is a process that involves the conversion of a nitrile functional group (-CN) to a carboxylic acid functional group (-COOH).
In this reaction, the nitrile is typically reacted with an acid or base in the presence of water to produce an amide intermediate, which is then further hydrolyzed to form the carboxylic acid.
For more question on Grignard carboxylation click on
https://brainly.com/question/9322175
#SPJ11
What type of change occurs at the molecular level?
Answers
When two or more molecules interact, chemical changes take place at the molecular level.
What transpires during a chemical change at the molecular level?The molecules in the reactants interact during a chemical reaction to create new compounds. No new material is created during a physical change, such as a state shift or dissolution. You may also assert that no atoms are generated or destroyed during a chemical reaction, so explain this.
How do molecular shifts in phase happen?The intermolecular interactions between the water molecules are weakening at the molecular level. The water molecules have access to enough energy from the heat to repel these forces. Intermolecular forces are either increased or decreased after every phase shift.
To know more about molecules interact visit:-
https://brainly.com/question/13770836
#SPJ1
If 1 g of acetanilide (molecular mass is 135. 17 g/mol) is used, how much (in mol) of nitronium ion do you need?
Answers
0.0074 mol of nitronium ion is needed to react with 1 g of acetanilide
To determine the amount of nitronium ion needed for the reaction with 1 g of acetanilide, we will first calculate the moles of acetanilide and then apply stoichiometry.
Given that the molecular mass of acetanilide is 135.17 g/mol, we can calculate the moles of acetanilide:
moles = mass / molecular mass
moles = 1 g / 135.17 g/mol ≈ 0.0074 mol
Now, we need to determine the stoichiometry of the reaction between acetanilide and nitronium ion. Assuming the reaction is a 1:1 ratio (i.e., one mole of acetanilide reacts with one mole of nitronium ion), the amount of nitronium ion needed would be the same as the moles of acetanilide.
Thus, approximately 0.0074 mol of nitronium ion is needed to react with 1 g of acetanilide. Remember to consider the reaction's stoichiometry when applying this calculation to other scenarios or chemical reactions.
Know more about stoichiometry here:
https://brainly.com/question/14420683
#SPJ11
consider the following polymer (pva) and potential-cross linking agent (boric acid). what type of intermolecular forces is likely to sustain cross-linking of polymeric chains in this system?
Answers
The cross-linking of PVA and boric acid is sustained by a combination of covalent and non-covalent interactions, including hydrogen bonding and van der Waals forces. These interactions lead to the formation of a stable, three-dimensional network structure that has a range of potential applications, including in the development of new materials with unique properties.
Polyvinyl alcohol (PVA) can form cross-linked networks when reacted with boric acid. The cross-linking is due to the formation of borate ester linkages between PVA chains and boric acid molecules. The formation of these linkages is facilitated by a combination of covalent and non-covalent interactions, including hydrogen bonding and van der Waals forces.
Hydrogen bonding is a particularly important intermolecular force that plays a key role in the formation and stability of the cross-linked PVA network. PVA contains hydroxyl (-OH) groups along its polymer chains that can form strong hydrogen bonds with the borate groups on boric acid molecules. This interaction leads to the formation of a three-dimensional network structure that is stabilized by the formation of multiple hydrogen bonds between adjacent PVA chains and boric acid molecules.
Van der Waals forces also contribute to the stability of the cross-linked network. These forces arise from the fluctuating dipoles in atoms and molecules and are responsible for the attraction between non-polar species. In the PVA-boric acid system, van der Waals forces between the polymer chains and boric acid molecules help to stabilize the cross-linked network.
For such more questions on Cross-linking of PVA:
https://brainly.com/question/13247684
#SPJ11
How many liters of 2.07 M sulfuric acid are needed to make 57 milliliters of a 0.58 M solution of sulfuric acid?
**Round to FOUR places after the decimal.
Answers
We need 0.0161 liters of the 2.07 M sulfuric acid solution to make 57 milliliters of a 0.58 M solution of sulfuric acid.
To solve this problemWe need to use the formula:
C1V1 = C2V2
Where
C1 is the concentration of the initial solutionV1 is the volume of the initial solutionC2 is the concentration of the final solutionV2 is the volume of the final solutionWe want to find the volume of the 2.07 M sulfuric acid solution needed to make 57 milliliters of a 0.58 M solution. Let's plug in the values we know:
2.07 M * V1 = 0.58 M * 57 mL
Simplifying the equation, we get:
V1 = (0.58 M * 57 mL) / 2.07 M
V1 = 16.0874 mL
To convert the volume to liters, we divide by 1000:
V1 = 16.0874 mL / 1000 mL/L
V1 = 0.0161 L
Therefore, we need 0.0161 liters of the 2.07 M sulfuric acid solution to make 57 milliliters of a 0.58 M solution of sulfuric acid.
Learn more about concentration here : brainly.com/question/28564792
#SPJ1
what happened to the cell potential when you added aqueous ammonia to the half-cell containing 0.001 m cuso4? how does ammonia react with copper ions in aqueous solution? (think back to coordination complexes in exp
Answers
When aqueous ammonia is added to the half-cell containing 0.001 M CuSO4, the cell potential is likely to change. The reason for this is that ammonia can form coordination complexes with copper ions, which can affect the concentration of copper ions in the solution, and hence the concentration gradient that drives the redox reaction in the cell.
Ammonia can react with copper ions in aqueous solution to form a series of coordination complexes. The most common complex is Cu(NH3)42+, which is a tetraamminecopper(II) complex. The formation of this complex reduces the concentration of free Cu2+ ions in solution, which can shift the equilibrium of the redox reaction in the cell.
If the reduction half-reaction is Cu2+ + 2e- → Cu, the addition of ammonia can reduce the concentration of Cu2+ ions in the solution and shift the equilibrium to the left, decreasing the cell potential. On the other hand, if the oxidation half-reaction is Cu → Cu2+ + 2e-, the addition of ammonia can increase the concentration of Cu2+ ions and shift the equilibrium to the right, increasing the cell potential.
Learn more about aqueous ammonia
https://brainly.com/question/14672082
#SPJ4